Living donor liver transplantation in India
Liver transplantation is undoubtedly one of the most successful innovations in the medical field over the last 50 years. Although it took 40 years for India to reap the benefits of this innovation, the current rate of growth of liver transplantation in the country is a clear testimonial to the success of this procedure in India.
History of liver transplantation in India
The Human Organ Transplantation Act was passed in India in 1994 following enormous effort by pioneers like Prof. S. Nundy and this marked the beginning of transplantation in a regulated manner in India. The era of Liver transplantation in India over the last 2 decades can be broadly divided into two phases—1995 to 2004 and 2005 to 2015. The first deceased donor liver transplant (DDLT) in India was done in 1995 and was unsuccessful. This was followed by a few unsuccessful attempts until the first successful DDLT in 1998 and shortly thereafter by the first successful Living donor liver transplant (LDLT) in November 1998 both performed by Rajashekar (1). The first era saw 131 transplants in total (DDLT and LDLT) done in 15 centers (2) and the second era has seen a progressive increase every year and a phenomenal growth over the last 2 years with close to 1,200 Liver transplants done in the year 2014 alone. This growth encompasses 85% LDLT and 15% DDLT. There is an interesting geographical distribution to this LDLT/DDLT divide as well. Programs in the north of India do LDLT predominantly (about 97% of the transplants performed) and programs in the south (of which we are one) do a combination of LDLT and DDLT (our program has a 70/30 distribution and others in the south have a higher proportion of DDLT). The initial failures led to introspection and a multi-pronged, comprehensive approach applying lessons learnt from the west in setting up units with adequate infra structure, multi-disciplinary specialists team with exposure and training in liver transplant and choosing the right patient in the early phase of a new program. As the implementation of deceased donation was facing hurdles in India during the early part of the second era, the knowledge imbibed from the technical advancement in LDLT from countries in the east like Japan, Korea and Hong Kong by transplant surgeons in India paved way for the successful launch of liver transplant programs in India, majority of them doing LDLT (3). Sir Ganga Ram hospital in Delhi was the seat of growth for LDLT in India from 2001 to 2009 (4). The focus on a multidisciplinary team has had a great impact on the success of transplant in India. The good outcomes of LDLT was a positive feedback for the establishment of liver transplant as a successful treatment option for end stage liver disease among the gastroenterologists, a medical fact which was long established in the west. At the time of this article being written, there are about 30 functioning liver transplant centers in India and a few more in the process of setting up a program. Five large volume centers do more than 100 transplants a year.
India has now emerged as the regional transplant centre for South East Asia. About 25% to 30% of the total transplants per year are performed on patients from other countries. Most countries from which patients are referred to India have a population and transplant need that will make it nonviable to set up a transplant unit for themselves and as a result the government opts to sponsor their patients for treatment abroad. A second scenario also exists where our neighboring countries are in the early phase of growth of transplant in their countries and hence the sick/high risk patients are referred to India. As the Human Organ Transplant Act (HOTA) of India allows for foreigners to receive deceased donor organs in India only if there is no suitable Indian recipient available for the organ, it practically does not offer a DDLT option to foreigners. This in turn contributes to the increase in LDLT in India as all the overseas patients will have a LDLT from a donor who is a relative of the recipient.
Technical innovations and advancement
Innovations are an integral part of growth and LDLT in India has followed the norm. Organ shortage is a universal problem in transplantation and ways to tackle it has always been an area for innovation. Here are a few strategies followed by the Indian programs to overcome this. The large number of transplants in India allow for the possibility of paired exchange either for size mismatch or for blood group incompatibility. In addition, ABO incompatible transplants using cascade plasmapheresis as a cost effective and useful method to reduce antibody titers have been performed by Tiwari et al. (5). Domino transplants, as is well known, can help overcome donor shortage. Domino auxiliary transplant by swapping portions of the liver between two patients with metabolic disease is a novel variation and has been successfully demonstrated for the first time in the history of transplantation by Govil et al. (6). Good outcomes with modified medical regimens in the management of hepatitis B post-transplant without immune globulins and with just anti virals have been established by Wadhawan et al. (7). This provides a very cost effective option to the Indian patients as a majority of them are self-funded. Other technical advancements like technique of arterial anastomosis in auxiliary LDLT, use of recanalised umbilical vein graft for anterior segment reconstruction, middle vein clamp technique to determine need for anterior segment reconstructions have been widely used and documented from programs in India (8,9).
These innovations establish the maturity and technical expertise achieved in LDLT in India. Pediatric transplant definitely calls for more surgical and intensive care expertise and the number of pediatric transplants done in India in 2014 was around 65 with our program contributing to 42 pediatric transplants. At this rate, India is soon expected to be one of the largest pediatric transplant centers in the world. This growth in pediatric transplantation with incorporation of several innovative techniques mentioned above was possible due to the significant percentage of metabolic disease etiology among children like Crigler-Najjar syndrome, propionic acidemia and primary hyperoxaluria (combined liver and kidney transplant) where an opportunity for implementation exists and the contribution by Prof. Rela towards this has been enormous (6,9,10).
Liver transplant has promoted the influx of professionals in other allied specialties like intensive care, radiology (diagnostic and interventional), infectious disease, transfusion medicine etc. back to India following training at established centers in the west. Radiology plays a very vital part in LDLT in planning the type of graft in an accurate and safe manner. Outsourcing of this component to places like Mevis in Germany was utilised by almost all programs world over and still continues to be done in some parts. Whereas in India, the logistics did not allow for this outsourcing in an effective way and this led to improvement in imaging techniques to match the high standard required for LDLT and currently we are not aware of any program that would outsource their images from India for this purpose. The understanding of the variability in incidence and presentation of problems and complications in the local population as opposed to what is present in the literature from transplant experience in the west cannot be emphasized more than in the practice of infectious disease in India. The classical example of this is the incidence of tuberculosis in India in the pre and post-transplant patients and the presentation of acute liver failure induced by anti-tuberculosis treatment whereas the experience from the west is quite limited (11).
Assessment of recipients for transplantation also has unique challenges in India. India has the largest population of diabetic patients in the world. The logical consequence to this is the increasing incidence of NASH as the etiology for ESLD. Hence cardiac evaluation in this sub group of patients results in encountering higher number of patients with coronary artery disease and the timing and modality to treat the coronary artery disease in patients with chronic liver disease is a complex decision.
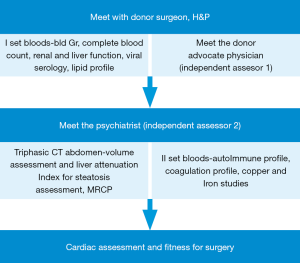
On the other hand, donor assessment and selection continues to follow a strict protocol. In spite of quite adventurous reports from the east about extended criteria living donor transplant from one septuagenarian to another (12), most centers in India are quite conservative in the context of expanding the criteria for living donor. The protocol followed in our program is a stepwise assessment as detailed in Figure 1 and the criteria for donor suitability is given in Table 1. Exceptions to the criteria are accepted only after extensive discussion in the pre transplant meeting. As the transplant population is a heterogeneous group from India, Middle east and south east Asia, donor screening is quite extensive and includes tests which are more relevant to some donors than the others based on epidemiology (e.g., G6PD deficiency is more common in the Middle East). Our experience with managing donors with varying degrees of coagulation disorders has accumulated over the last few years. Most of these conditions are not screened routinely by most LDLT programs worldwide as shown by very few reports in the literature pertaining to dealing with such situations. The acceptance rate of donors has been close to 64%, rejections have been secondary to medical reasons or social reasons.

Full table
The evolution of LDLT in India coincided with the time when Indian transplant scenario was striving to re-emerge from the reputation of “commercialization” of transplant from the kidney transplants performed in the earlier decades. This led to stringent rules for LDLT and has led to the establishment of transparent process where authorization committees have been established in all states in India (13). Every Indian patient who has a donor other than first degree relative is subjected to the committee approval and every foreign national undergoing a LDLT in India has to pass through the authorization committee with due documents establishing his donors and his origin and their relationship from his country (14). The process is more easy and streamlined better when the ministry of health (MOH) of a country has a ‘Memorandum of understanding’ with programs in India to perform transplant for their patients. This helps the Indian program in sharing the responsibility with the MOH in establishing the genuine relationship of the recipient-donor and also from the standpoint of follow up and care of the patient and donor after return to their country after transplant.
Techniques
Donor surgery continues to be done by the open technique in India. Dealing with the middle vein is dependent on program preference and each have their protocol (15). Recipient surgery is invariably done in the piggy back fashion and veno-venous bypass has been given up by and large. A remnant volume of 30% is acceptable and we aim to give a GRWR of 0.8. Blood banks in transplant programs are equipped to provide with blood and components and have the capacity to support even three transplants per day (as it occasionally happens in a program like ours when a DDLT happens on a day when two elective LDLT have been scheduled). Adjuncts like tissue sealants and factor seven preparations are also available in India over the years. Once again it helps to stress that the availability of such technological advances was ushered in by the increase in volume of liver transplants in India. Both the recipient and donor are observed in specialized transplant ICU staffed with Intensive care physicians and transplant trained nurses. The hospital stay is around 2 weeks and patients are required to follow up as an outpatient in the transplant clinic for a minimum of 6 to 8 weeks when they are referred back to the general practitioner. The immune suppression monitoring continues to be done by the transplant program through email in liaison with the local physician.
The immune suppression is quite variable from program to program, as it is worldwide, and it is well known the art of immune suppression can be mastered in several ways. The underlying logic though among all Indian programs is that Indians need lower immune suppression. This belief has probably stemmed from the fact that we see infection more often than rejection in India (16). Induction with interleukin blockers or antibody preparation are seldom used with the exception of ABOi and combined liver and kidney transplants. The prevalence of diabetes and the consequent diabetic nephropathy has promoted usage of renal sparing immune suppression protocols by using mycophenolate mofetil in the standard regimen.
Outcome
The outcome following transplant in India has improved over the years with increasing experience and the 1-year mortality quoted in 2011 varied from 10% to 24% (17,18). Current outcomes from most centers claim a 1-year survival of 85% to 90% as presented in the recently concluded conference in Chennai, India on LDLT (17). The higher incidence of mortality secondary to infection in the initial phase plays an important factor. The experience at our program has shown that patients from India fair poorly as compared to patients from overseas and also patients who undergo DDLT fair poorly compared to LDLT. The root cause for both these observations can be narrowed down to one factor that patients from India are referred late for transplant after multiple episodes of infections (SBP, UTI, malnourished) or deteriorate waiting for a DDLT. In DDLT, the donor maintenance also plays a very important factor and a uniform protocol is yet to be established.
Peculiar problems seen in India
Referral for transplant
Liver transplantation in India is focused primarily in the private sector. The attempts at public sector have met with limited success. This factor has a double impact on transplantation—one is the medical aspect and the other is the financial aspect. The healthcare structure in the private sector does not follow a specific referral pattern and this limits the opportunity to provide uniform level of primary, secondary and tertiary care. As a result, awareness among general practitioners about the indications and success of transplant plays a vital role in timely referral. Mismanagement of patients with hepatitis B and C and complacency in dealing with NASH is a major contributor for late referrals. Alcoholic liver disease in its presentation is no different from the western world. High MELD at presentation to a transplant centre is not uncommon. Added to this is the fact that most of these patients have had multiple prior admissions to hospitals and have been exposed to antibiotics. This sets the stage for multi drug resistant infection even before the transplantation and increases the cost of treatment by several fold. The financial aspect is that the cost of Liver transplant in India is about 50,000 USD. It equates to only about one fifth of the cost in the western world and hence works out economical for patients who travel from abroad and are able to get quality care and good outcome for a better price. But that rationale does not apply to the majority of Indian patients as the funding for the treatment is done by the patient themselves in 95% of the instances. Insurance or government funding is an exception. This complicates the decision further in pediatric patients with liver disease. When the infants are diagnosed very early in life, the financial implications of a lifelong commitment do weigh heavily in making a decision to proceed with transplantation. The need for establishment of successful transplant programs in the public sector cannot be overemphasized. There is also a huge scope for public private partnership towards the achievement of this target.
Donor options
As alluded to earlier, diabetes is prevalent in India as is lean NASH. This limits the suitability of a family member being a donor. The next limitation that is perceived is in the form of social implications to a female donating to her family member, be it an unmarried or a married woman, both having different ramifications. Analysis of data from our centre has revealed 25.5% of the live donors to be the recipient’s offspring and 40% of them were daughters and in most instances they were not the first choice. We are yet to collect data on whether this had any social implications or quality of life issues in their lives. In patients with metabolic disease, the living donor has to be evaluated very carefully to look for clinical/biochemical evidence of the disorder in the recipient as most of these have a familial transmission of the genetic trait.
Lack of database
As the reader might have noticed that the data quoted throughout this article are from published data/from presentation at meetings or from personal communications and not from registry data. This is the next big challenge that transplant programs in India are working to overcome. As India is emerging as one of the largest centers performing LDLT, a registry is of paramount importance to authenticate and validate the numbers and experience quoted. Once again, as most programs are in the private sector and LDLT is the predominant form of liver transplant, there is as yet no central body like the UNOS to overlook all transplants in the country. Preliminary discussions which are now ongoing should mature into a registry in the near future. There have been seven reported donor deaths in India but no consolidated data of the overall morbidity has been published. There has also not been a uniform policy on process or reporting to be followed by the program in the event of a donor death and is left to individual responsibility (19).
Vision about the evolution and growth of LDLT in this region
Liver transplantation in India has led to huge progress in several aspects of medical care. Transplantation, as a specialty requires full time professionals working with multi-disciplinary team with great emphasis on the importance of team work. This concept has paved the way for establishment of several multi-specialty hospitals and all of them striving towards international accreditations in order to cater to patients from abroad. This has definitely increased the standard of medical care in India over the years. It has also led to a “reverse brain drain” of sorts in encouraging Indian doctors trained abroad to return to the home country where the opportunity is now available to practice and provide quality care.
LDLT has also refined hepatobiliary surgery in the country to a significant extent. Surgeons trained at LT centers have a different perspective to non-transplant liver surgeries and are able to provide excellent outcomes (20). Training for aspiring liver transplant surgeons was not available in India until 7 years ago and most of us from that period received our DDLT training from the west and LDLT training from the east. Today, India is able to provide training in both LDLT and DDLT with university approved fellowship programs and most programs have international trainees and visitors.
LDLT programs in India feel a strong social responsibility towards developing DDLT in the country. The success of LDLT has definitely reposed faith among medical professionals on the science of transplantation and hence see a purpose in promoting deceased donation. The number of deceased donation is showing a steady progress over the years and stands at 1.3 per million in one state of Tamil Nadu in south India which performs the maximum number of DDLT in the country (21,22). A few other states have also taken the cue from Tamilnadu and are gradually increasing the deceased donation rate. Nevertheless, a rough estimate of the need for LT in India is ten to twelve thousand per year and the current number fulfils only 10% of the requirement. LDLT in India is expected to expand to many more centers along with DDLT. Though the idealistic expectation is for the supply of DDLT to one day suffice the demand for LT in India, the realistic scenario is for LDLT to be available to satisfy the demand for a long time to come.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Poonacha P, Sibal A, Soin AS, et al. India’s first successful pediatric liver transplant. Indian Pediatr 2001;38:287-91. [PubMed]
- Kakodkar R, Soin A, Nundy S. Liver transplantation in India: its evolution, problems and the way forward. Natl Med J India 2007;20:53-6. [PubMed]
- Varma V, Mehta N, Nundy S. The History of Liver Transplantation in India. In: Nundy S, editor. Liver Transplantation in India. India: Elsevier, 2011.
- Kumaran V. Liver Transplantation at Sir Ganga Ram Hospital. The Gaga Ram Journal 2011;1:28-31.
- Tiwari AK, Pandey P, Aggarwal G, et al. Cascade plasmapheresis (CP) as a preconditioning regime in ABO-incompatible live related donor liver transplants (ABOi-LDLT). Transplant Res 2014;3:17. [PubMed]
- Govil S, Shanmugam NP, Reddy MS, et al. A metabolic chimera: Two defective genotypes make a normal phenotype. Liver Transpl 2015;21:1453-4. [PubMed]
- Wadhawan M, Gupta S, Goyal N, et al. Living related liver transplantation for hepatitis B-related liver disease without hepatitis B immune globulin prophylaxis. Liver Transpl 2013;19:1030-5. [PubMed]
- Singh A, Soin AS, Kakodkar R, et al. Recanalized umbilical vein as a conduit for anterior sector venous outflow reconstruction in right lobe grafts. Surgery 2007;141:830. [PubMed]
- Rela M. Technique of hepatic arterial anastomosis in living donor pediatric auxiliary partial orthotopic liver transplantation. Liver Transpl 2013;19:1046-8. [PubMed]
- Narasimhan G, Govil S, Rajalingam R, et al. Preserving double equipoise in living donor liver-kidney transplantation for primary Hyperoxaluria Type 1. Liver Transpl 2015;21:1324-6. [PubMed]
- Olithselvan A, Rajagopala S, Vij M, et al. Tuberculosis in liver transplant recipients: experience of a South Indian liver transplant center. Liver Transpl 2014;20:960-6. [PubMed]
- Kim SH, Kim YK, Lee SD, et al. Successful living donor liver transplantation between septuagenarians. Am J Transplant 2015;15:274-7. [PubMed]
- Shroff S. Legal and ethical aspects of organ donation and transplantation. Indian J Urol 2009;25:348-55. [PubMed]
- Gupta S. Living Donor Liver Transplant is a Transparent Activity in India. J Clin Exp Hepatol 2013;3:61-5. [PubMed]
- Rela M, Kota V, Shanmugam V, et al. Middle hepatic vein to middle hepatic vein anastomosis in right lobe living donor liver transplantation. Liver Transpl 2013;19:229-31. [PubMed]
- Varghese J, Reddy MS, Venugopal K, et al. Tacrolimus-related adverse effects in liver transplant recipients: its association with trough concentrations. Indian J Gastroenterol 2014;33:219-25. [PubMed]
- Sudhindran S. Experience at Amrita Institute of Medical Sciences, Kochi, kerala. In: Nundy S, editor. Liver Transplantation in India. India: Elsevier, 2011.
- Soin AS. Our Journey of Liver Transplantation at Sir GangaRam and Medanta Hospitals: A rewarding decade of trials and tribulations. In: Nundy S, editor. Liver Transplantation in India. India: Elsevier, 2011.
- Reddy MS, Narasimhan G, Cherian PT, et al. Death of a living liver donor: opening Pandora’s box. Liver Transpl 2013;19:1279-84. [PubMed]
- Mistry JH. Impact of Living Donor Liver Transplantation Experience on Liver Resection. J Transplant Technol Res 2015;5:145.
- Abraham G, Reddy YN, Amalorpavanathan J, et al. How deceased donor transplantation is impacting a decline in commercial transplantation-the Tamil Nadu experience. Transplantation 2012;93:757-60. [PubMed]
- Narasimhan G. Rela M. In: Nundy S, editor. Liver Transplantation in India. India: Elsevier, 2011.