The evolution of anterior sector venous drainage in right lobe living donor liver transplantation: does one technique fit all?
Introduction
Transplantation of the right lobe (RL) is a major achievement in living donor liver transplantation (LDLT), which has made liver transplantation possible in some countries, while in others, it has created a significant increase in graft supply. However, RL LDLT is a technically demanding procedure, not only because of the high frequency of anatomical variations (1,2), but particularly because of the unique functional anatomic characteristics of hepatic venous drainage (3). Failure to fully understand the functional anatomy of the hepatic veins may lead to graft dysfunction and small-for-size syndrome (SFSS), even in a liver graft of adequate size (4). More importantly, it may also jeopardize donor safety because of a possible postoperative venous congestion of anterior sector (AS) of the liver remnant (5).
A good hepatic venous outflow constitutes one of the four basic principles of a technically successful LDLT procedure (the others being adequate graft volume, sufficient inflow, and secure bile duct anastomosis) (3,6). Only after recognizing the functional anatomy of the liver, the outcomes of RL LDLT have become comparable (7), even superior to that of DDLT (8). From the hepatic venous outflow perspective, the issue of whether the AS of the RL graft should or should not be routinely drained has been controversial since the initiation of adult-to-adult LDLT (9).
Our transplant program represents one the largest LDLT programs in the western world. We have gained significant experience since late 1990s when our group started LDLT practice. With this experience, we have seen an evolution of our technique as well as approach to anatomical issues related to venous drainage of RL LDLT. The aim of this 10-year, single-center, retrospective cohort study is to review the evolution of hepatic venous outflow reconstruction technique in RL grafts and evaluate the impact of routine AS drainage strategy on the outcome.
Patients and methods
Between July 2004 and December 2014, we performed 582 primary RL LDLT at Liver Transplantation Unit of Istanbul Bilim University, Florence Nightingale Hospital, Istanbul, Turkey. Patient demographics, operative findings, and follow-up data were retrospectively analyzed using a prospectively maintained database. The minimum evaluation requirements for potential living liver donors included age over 18, absence of any history of medical condition that would significantly increase the perioperative risks, blood group compatibility with the recipient, and relatedness to the recipient within the fourth degree of consanguinity. All potential living unrelated donors and those beyond the fourth degree of consanguinity were evaluated by the central Ethical Commission of Ministry of Health.
Study design
The surgical technique of hepatic venous outflow reconstruction of the RL grafts was retrospectively extracted: the retrieval and reconstruction of MHV, reconstruction of segment 5 and/or 8 veins and accessory inferior hepatic veins (AIHV), and utilization of cryopreserved homologous and/or synthetic (dacron or polytetrafluoroethylene) vascular grafts were recorded. The technique of inferior vena cava (IVC) clamping (total vs. side) and the presence of splenic artery ligation (SAL) were also evaluated. The cases were divided into 3 consecutive periods with distinctive AS venous outflow reconstruction techniques. In the first period, AS drainage was utilized in half of the cases and inclusion of MHV in the graft was the dominant technique (Era 1). In the second period, the number of MHV retrievals, as well as the utilization of total IVC clamping significantly decreased and AS drainage was performed more selectively using cryopreserved homologous grafts (Era 2). In the last period, MHV retrieval was abandoned and a segment 5 and/or 8 oriented AS drainage strategy was implemented. These three groups were compared in terms of patient and donor demographics, surgical characteristics and short-term outcome.
Selection criteria for donors
All potential living donors underwent a three-step evaluation. In step 1, the medical history and the psychosocial status were assessed together with complete biochemical and serologic profile. In step 2, radiologic assessment was performed using tri-phasic computer tomography (CT) and magnetic resonance (MR) cholangiography. In step 3, medical evaluation was completed with the assessment of cardiac and respiratory functions, and thrombophilia screening for factor 2 and factor 5 Leiden mutations. Liver biopsy was performed selectively.
For the RL donors, the same selection criteria [i.e., age limit, degree of steatosis, graft-to-recipient weight ratio (GRWR), and acceptance of vascular and biliary variations] were used throughout the study. The overall anesthetic or surgical technique for donors and recipients (except for AS drainage variations) did not change significantly.
Surgical technique
All of the donor hepatectomies were performed or directly supervised by the same senior surgeon, while all of the recipient operations were performed by the two transplant surgeons.
Donor hepatectomy was performed as previously described (10). An upper midline incision with or without subcostal extension was used for all donors. A Cavitron ultrasonic surgical aspirator (CUSA) was used for parenchymal dissection. Intraoperative ultrasound (IOUS) was used to delineate the hepatic venous anatomy.
During mobilization of the RL, all AIHVs of greater than 5 mm were clipped and divided for a possible back-table reconstruction. Before division of right hepatic artery, 1,000 IU heparin is administered intravenously. Once the graft is removed, it was perfused through portal vein with histidine-tryptophan-ketoglutarate solution (Custodiol®) on the back table.
The recipient operation is begun shortly after the start of the donor operation and after the eligibility of the donor liver is confirmed. The recipient hepatectomy is performed using the piggyback technique. Venovenous bypass was not utilized in any case and temporary portocaval shunt was used selectively (i.e., fulminant hepatic failure). After division of the hepatic arteries and the bile duct close to the hepatic parenchyma, recipient’s liver is fully mobilized and recipient hepatectomy is completed by the time the donor graft is available for transplantation.
Era 1 (MHV dominant AS drainage)
In the first 119 cases, which were performed between July 2004 and March 2008, the main strategy for AS drainage was MHV retrieval (50.4%). When MHV is procured with the graft, segment 5 and 8 veins are preserved and parenchymal dissection is performed on the left border of the MHV. The MHV orifice is reconstructed directly to RHV orifice to create a single triangular opening, as described by Lo et al. (11). In MHV (−) grafts, segment 5 and 8 venous branches are clipped and divided, and parenchymal dissection is performed on the right border of the MHV. During the portal perfusion in the back table, the function of segment 5 and 8 veins is reevaluated for AS congestion while they are clipped, and for initial outflow volume after the clips are removed. In this period, most (97.5%) of the hepatic vein reconstructions were performed under total IVC clamping. In MHV (−) grafts, the recipient RHV orifice was always enlarged with an anterior slit towards its junction with the IVC, creating a triangular opening approximately 1.5 times the diameter of the graft RHV.
Era 2 (Selective AS drainage)
In the next 391 patients, who underwent RL LDLT after March 2008, the number of MHV retrievals significantly decreased (15.9%), mostly because of the concerns about the need for recipient total IVC clamping in the MHV (+) grafts. The use of recipient total IVC clamping also decreased considerably (32.2%) in this period. The selection of recipient IVC clamping is made according to venous anatomy of the graft and the recipient IVC. For separate drainage of segment 5 and/or 8 veins, cryopreserved iliac vein grafts were the most common material used (25.6%). The AS drainage is performed selectively (35%) according to GRWR, the size of segment 5 and 8 veins, remnant liver volume, and the presence of segment 4b vein in the donor, as described previously (12).
Era 3 (Routine AS drainage)
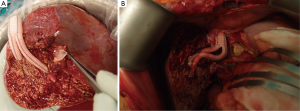
In the last 72 patients, who underwent RL LDLT after January 2014, we made major modifications in our operative protocol: (I) we completely abandoned complete MHV harvest in the donors; (II) we performed AS drainage whenever possible, either by partial MHV retrieval or by separate drainage of all sizeable (≥ 5 mm) segment 5 and/or 8 veins. In both techniques, AS veins are anastomosed to the common stump of the recipient middle and left hepatic veins (MHV-LHV) using synthetic grafts instead of cryopreserved homologous grafts (Figure 1); (III) we started performing SAL in all RL grafts, in which the post reperfusion IOUS showed portal flow of ≥250 mL/min/100 g liver tissue; (IV) in this era, liver mobilization during recipient hepatectomy is performed exclusively under early portal clamping to decrease blood loss; (IV) during graft implantation, venous reconstruction is performed under side clamping of IVC, whenever possible (90.3%).
Statistical analysis
For statistical analyses, IBM SPSS Statistics for Macintosh, Version 22.0 (IBM Corp. in Armonk, NY) was used. Continuous variables are expressed as means and standard deviations or medians and interquartile ranges and were analyzed with one-way analysis of variance (ANOVA). Pearson correlations were used to analyze the degree of linear dependence between 2 continuous variables. Categorical variables are expressed as numbers and percentages and were analyzed with the chi-square test. The Kaplan-Meier method was used for construction of survival curves, and they were compared by Wilcoxon test. All reported P values are 2-sided, and P<0.05 was considered statistically significant.
Results
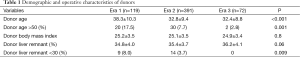
The demographic and operative characteristics of the groups are presented in Table 1. The mean donor age significantly decreased after the first era (38.3±10.3 in Era 1 vs. 32.8±9.4 in Era 2 and 32.4±8.8 in Era 3, P<0.001). The proportion of donors older than 50 years also decreased significantly (17.5% in Era 1 vs. 7.7% in Era 2 and 2.8% in Era 3, P=0.001). Meanwhile, the mean donor liver remnant percentage increased over time (34.8±4.0 in Era 1 vs. 35.4±3.7 in Era 2 and 36.2±4.1 in Era 3), where the difference between the groups showed a marginal statistical significance (P=0.06). The proportion of donors with a small remnant liver (<30%) also significantly decreased over time (8.0% in Era 1 vs. 3.7% in Era 2 and 0% in Era 3, P=0.009).
Full table
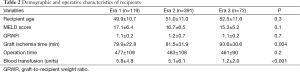
There was no statistically significant difference between the groups in terms of recipient age, biological MELD score at transplant, GRWR, and operative time (Table 2). Mostly due to early portal clamping before liver mobilization, blood transfusion requirement decreased significantly from a mean of 5.8±4.8 units in Era 1 and 5.1±6.1 units in Era 2 to 1.2±2.0 units in Era 3 (P<0.001).
Full table
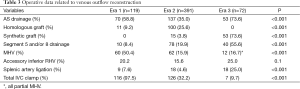
Operative data regarding venous reconstruction technique is given in Table 3. Venous drainage patterns included MHV drainage in 128 patients (22.0%) and segment 5 and/or 8 drainage in 134 patients (23.0%). The rate of AS venous drainage varied from 58.8% in Era 1 and 35.0% in Era 2 to 73.6% in Era 3 (P<0.001). Meanwhile, the rate of MHV drainage decreased from 50.4% in Era 1 to 15.9% in Era 2 and 16.7% in Era 3 (P<0.001). Full length MHV dissection was completely abandoned and replaced by partial MHV retrieval in Era 3. In 21.4% of patients with MHV drainage and 38.8% of patients with segment 5 and/or 8 drainage, AIHV drainage was also performed. In Era 3, routinely performed venous reconstructions significantly prolonged the back table procedures. Thus, graft total ischemia time increased significantly from a mean of 79.9±22.8 min in Era 1 and 81.5±31.9 min in Era 2 to 93.6±30.6 min in Era 3 (P=0.004). However, in grafts with any type of AS drainage vs. grafts without AS venous reconstruction, postoperative day 1 (POD1) AST (279±290 vs. 229±141 U/L, P=0.02), ALT (293±388 vs. 224±147 U/L, P=0.02), and POD7 total bilirubin levels (7.1±7.6 vs. 5.6±5.1 mg/dL, P=0.01) were significantly lower.
Full table
The type of vascular graft, which is used for the reconstruction of AS drainage veins changed over time. While the graft of choice was cryopreserved iliac vein graft in Eras 1 and 2, we completely abandoned the use of homologous grafts in the third era, where we exclusively used Dacron grafts in 53 patients. Dacron graft patency was investigated in 73% (n=38) of these patients using either Doppler ultrasound (DUS) (n=25) and/or CT angiography (n=29). Dacron graft was patent in 32 of 38 patients (84.2%) in a median time of 37 (10.0−97.5) days after LDLT. In 6 patients with AS venous outflow obstruction, no significant clinical consequence was observed.
In the third Era, the frequency of SAL increased significantly from 7.6% in Era 1 and 3.6% in Era 2 to 25% in Era 3 (P<0.001). In cases with SAL, mean portal flow decreased significantly from 2,520±753 to 1,674±536 mL/min (P=0.01).
Most importantly, perioperative mortality rate significantly decreased in the routine AS venous drainage era (15.1% in Era 1 and 8.7% in Era 2 vs. 2.8% in Era 3, P=0.01) (Table 4). In Era 3, perioperative mortality was seen only in 2/72 patients, and one other patient underwent early retransplantation because of graft dysfunction. One-year patient survival rate was also significantly higher in the third era (79.6% in Era 1 and 86.1% in Era 2 vs. 92.1% in Era 3, P=0.002) (Figure 2).

Full table

Discussion
In this retrospective study, we examined the evolution of hepatic venous outflow reconstruction in RL grafts over the last decade. During the study period, neither the selection criteria for RL donors (i.e., age, GRWR, and acceptance of vascular and biliary variations), nor the surgical technique (i.e., donor hepatectomy, recipient hepatectomy, and arterial, portal, and biliary anastomoses) changed significantly, however, the short-term recipient outcomes significantly improved with the routine AS drainage strategy.
We have previously reported that recipients of RL grafts without AS drainage showed a significantly lower average POD7 regeneration rate in CT volumetry (12). Yamamoto et al. (13) have also shown that, in RL grafts without AS drainage, tissue congestion was observed in 88% of segments 5 and 85% of segments 8 during the first postoperative month by MR imaging. The consequences of venous congestion in the AS of RL graft may result not only in impaired graft regeneration (14), but also immediate liver dysfunction (4), graft rupture (15), and graft loss.
Today, most of the high-volume LDLT centers recognize the AS venous drainage as a prerequisite for RL grafts (3,16). However, this concept varies from absolute inclusion of the MHV in the graft in every case to selective drainage of segment 5 and/or 8 veins via interposition vascular grafts. In our initial experience with RL LDLT, AS venous drainage was mostly performed by total MHV procurement (12). In Era 1, half of the RL grafts included the MHV, and individual segment 5 and/or 8 drainage was performed only in 8% of the grafts. In this period, the MHV orifice was always reconstructed directly to the RHV orifice where venous reconstruction required to be performed under total IVC clamping for optimal drainage. However, total IVC clamping was not well tolerated in patients with hemodynamic instability and those with pre-existing renal dysfunction. Meanwhile, we also began to appreciate the negative impact of total MHV procurement on donor outcomes (10). After 2008, our policy changed in a way that, total MHV procurement declined significantly from 50.4% to 15.9% and the rate of total IVC clamping dropped from 97.5% to 32.2%. The most frequent type of vascular graft in this era was cryopreserved iliac vein. Unfortunately, the rate of AS drainage also dropped from 58.8% to 35.0%, mostly because of the limited source of cryopreserved homologous grafts. Because of the patency and infection issues related with PTFE grafts, we hesitated to use synthetic grafts for AS drainage (17). Despite a significant reduction in perioperative mortality in Era 2 (from 15.1% to 8.2%), initial poor graft function was the most common cause of death and 77% of cases with perioperative mortality in this era did not have AS drainage.
In the beginning of 2014, we decided to start using Dacron grafts for AS drainage. This strategy provided an unlimited source of vascular grafts with an opportunity to perform AS drainage in every RL graft. With the help of the Y-shaped grafts, we performed separate segment 5 and/or 8 drainage in 55.8% of the cases. We also abandoned total MHV procurement technique for donor safety. In partial MHV procurement, the proximal segment of the MHV is kept in the donor to preserve any significant segment 4 veins draining into the MHV, as described in the Nakamura classification (18). As a result, despite the procurement of RL graft with the MHV, the risk of AS venous congestion of the remnant liver is completely eliminated. In addition, extension of the partial MHV with an interposition Dacron graft and anastomosing separately to the MHV-LHV junction under the side clamp provides a more physiologic reconstruction.
Even in the presence of AS drainage and an adequate graft volume, a high portal flow creates a sustained hyper dynamic injury, which may still result in a functional SFSS. A portal flow of 250 mL/min/100 g liver tissue has been accepted as the threshold for portal hyperperfusion in LDLT (19). In Era 3, we started to measure the portal flow using intraoperative Doppler ultrasonography. In all cases with post-reperfusion portal flow of ≥250 mL/min/100 g liver tissue, we performed SAL. Since Lo et al. (20) described the salvage of a small-for-size liver graft; SAL at the time of transplantation has been accepted as an effective strategy to prevent SFSS. In our study, we found that SAL provided an average 33% reduction in the portal flow. More importantly, of 45 cases we performed SAL in conjunction with the routine AS drainage, there was only 1 graft loss and no perioperative mortality.
This study has several limitations. First, it is retrospective in design and therefore potentially subject to systematic error and bias. Second, in addition to intraoperative management strategies, the younger donor age and the significantly lower number of elderly donors in Era 3 might have contributed to improved outcomes in the routine AS drainage period. A younger donor age has been proposed to ameliorate the effects of hyper dynamic portal flow (21) and is associated with an increased liver regenerative capacity (22). However, despite a significant difference in perioperative mortality rate between Era 2 and Era 3 (8.7% vs. 2.8%, respectively), donor age was identical in both groups, which minimizes the possibility of selection bias. In addition, significant reduction of red blood cell transfusion requirements by early portal camping during recipient hepatectomy might have also helped to reduce perioperative mortality. Third, median follow-up time in Era 3 is only 8 (range, 4−11.8) months, which is significantly lower than that of other groups. A longer follow-up is needed to truly determine both the safety and long-term efficacy of routine AS drainage strategy.
Because the study was designed to demonstrate the evolution of our approach and operative technique, there is an apparent era effect with improving outcomes with experience. However, the significant differences in outcome cannot be explained simply by the “learning curve” effect. The learning curve in LDLT has been described previously in a range of 15 to 50 cases (23-25). We started utilizing the technique of routine AS drainage with SAL after 510 cases of RL LDLT, which is well beyond every previous definition, including the 200 mark, which has been defined as the criteria for a high-volume center in LDLT (26).
Conclusions
Routine AS drainage via segment 5 and/or 8 veins using synthetic grafts is a technique to fit all RL grafts in LDLT. It depends on an appreciation of functional anatomy, is based mainly on hepatic venous configuration rather than complex and individual algorithms, and is universally applicable. Addition of SAL effectively prevents early graft dysfunction and significantly improves the outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nakamura T, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation 2002;73:1896-903. [PubMed]
- Ohkubo M, Nagino M, Kamiya J, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg 2004;239:82-6. [PubMed]
- Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant 2015;15:17-38. [PubMed]
- Lee S, Park K, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation 2001;71:812-4. [PubMed]
- Toshima T, Taketomi A, Ikegami T, et al. V5-drainage-preserved right lobe grafts improve graft congestion for living donor liver transplantation. Transplantation 2012;93:929-35. [PubMed]
- Lee SG. Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J Hepatobiliary Pancreat Surg 2006;13:131-8. [PubMed]
- Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:1226-36. [PubMed]
- Chan SC, Fan ST, Lo CM, et al. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg 2008;248:411-9. [PubMed]
- de Villa VH, Chen CL, Chen YS, et al. Right lobe living donor liver transplantation-addressing the middle hepatic vein controversy. Ann Surg 2003;238:275-82. [PubMed]
- Taner CB, Dayangac M, Akin B, et al. Donor safety and remnant liver volume in living donor liver transplantation. Liver Transpl 2008;14:1174-9. [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Hepatic venoplasty in living-donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation 2003;75:358-60. [PubMed]
- Dayangac M, Taner CB, Balci D, et al. Use of middle hepatic vein in right lobe living donor liver transplantation. Transpl Int 2010;23:285-91. [PubMed]
- Yamamoto H, Maetani Y, Kiuchi T, et al. Background and clinical impact of tissue congestion in right-lobe living-donor liver grafts: a magnetic resonance imaging study. Transplantation 2003;76:164-9. [PubMed]
- Akamatsu N, Sugawara Y, Kaneko J, et al. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation 2003;76:832-7. [PubMed]
- Marcos A, Fisher RA, Ham JM, et al. Emergency portacaval shunt for control of hemorrhage from a parenchymal fracture after adult-to-adult living donor liver transplantation. Transplantation 2000;69:2218-21. [PubMed]
- Fan ST, De Villa VH, Kiuchi T, et al. Right anterior sector drainage in right-lobe live-donor liver transplantation. Transplantation 2003;75:S25-7. [PubMed]
- Madden RL, Lipkowitz GS, Browne BJ, et al. A comparison of cryopreserved vein allografts and prosthetic grafts for hemodialysis access. Ann Vasc Surg 2005;19:686-91. [PubMed]
- Chan SC, Lo CM, Liu CL, et al. Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation. Liver Transpl 2004;10:755-62. [PubMed]
- Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl 2003;9:S36-41. [PubMed]
- Lo CM, Liu CL, Fan ST. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft-successful treatment with splenic artery ligation. Liver Transpl 2003;9:626-8. [PubMed]
- Sanefuji K, Iguchi T, Ueda S, et al. New prediction factors of small-for-size syndrome in living donor adult liver transplantation for chronic liver disease. Transpl Int 2010;23:350-7. [PubMed]
- Dayangac M, Taner CB, Yaprak O, et al. Utilization of elderly donors in living donor liver transplantation: when more is less? Liver Transpl 2011;17:548-55. [PubMed]
- Saidi RF, Elias N, Ko DS, et al. Live donor partial hepatectomy for liver transplantation: is there a learning curve? Int J Organ Transplant Med 2010;1:125-30. [PubMed]
- Broering DC, Wilms C, Bok P, et al. Evolution of donor morbidity in living related liver transplantation: a single-center analysis of 165 cases. Ann Surg 2004;240:1013-24; discussions 1024-6.
- Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl 2000;6:296-301. [PubMed]
- Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl 2013;19:499-506. [PubMed]


