Living donor liver transplantation in the USA
Introduction
In this article, we review the history of living donor liver transplant (LDLT), its current role in liver transplantation in the USA, statistics on its use, and data on its outcomes. We then discuss biliary complications and donor risk associated with the procedure.
History of LDLT in the USA
The first successful LDLT was performed by Strong et al. in Australia (1). In the USA, Broelsch et al. from the University of Chicago reported a series of 20 LDLT in pediatric patients in 1991 (2). The technical aspects of the procedure were refined with the center’s experience: the first three patients received left-lobe grafts without the middle hepatic vein, and the subsequent 17 patients received segment 2 and 3 grafts. Although the donors had some minor complications, all of them did well and the outcomes of the recipients were favorable. This series established the safety and feasibility of LDLT for children in the USA.
Once successful LDLT was reported in children, it was entertained in adults. LDLT became the procedure of choice in Japan, where deceased donor liver transplant (DDLT) was uncommon (3). In the USA, Emond et al. reported two adult patients with LDLT using left lobes (4). There were some concerns of a relatively high incidence of small-for-size syndrome with the left-lobe grafts, as reported by the group from New York (5). To provide the recipients with adequate liver volume, right-lobe grafts were considered. After the first adult-to-adult right-lobe liver transplant in the USA was performed by Wachs and his colleagues in 1997 (6), more centers performed LDLT with right-lobe grafts (7,8).
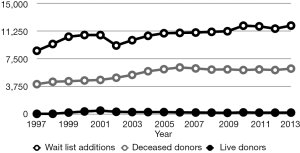
In the USA, DDLT has always been the main source of organs for patients listed for liver transplantation. Yet, with the well-recognized shortage of deceased donor livers compared with the number of patients on the waiting list for transplant, LDLT was increasingly offered as an option. In 1997, only one center performed adult LDLT, but by 2000, this number had increased to 38 centers, with 266 adult-to-adult LDLT (9). As the safety and outcomes of right-lobe LDLT became acceptable, there was a gradual increase in the LDLT with the peak of 524 in 2001, until 2002, when the rate decreased to 363 and reached a plateau to 252 in 2013 (Figure 1). This trend is a result of many factors, but may be due partly to a widely publicized death of a living donor (10). Although the rate of LDLT has not increased since that time, it remains an option to address the organ shortage, along with using expanded criteria for accepting donor grafts (11-14).
The role of LDLT in the USA
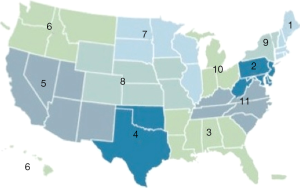
In the USA, deceased donor livers are allocated based on the 11 regions of the United Network of Organ Sharing (UNOS) (Figure 2). Each region is unique in the density of the population, the number of available organs, and the wait time for patients. The liver allocation system is strictly based on the model for end-stage liver disease (MELD) score (which ranges from 15 to 40), except in cases of status 1A (fulminant hepatic failure, primary nonfunction in the first week, and hepatic artery thrombosis in the first week). With the “sickest first” policy, deceased donor livers are allocated to the patients with the highest MELD scores (15). For patients with medical conditions that do not result in high MELD scores but may contribute to wait list mortality and therefore warrant allocation of livers, MELD exception points are given. These conditions include malignancies (e.g., hepatocellular carcinoma (HCC), hilar cholangiocarcinoma) and other medical conditions including hepatopulmonary syndrome, portopulmonary hypertension, familial amyloid polyneuropathy, primary hyperoxaluria, and cystic fibrosis. MELD exception points are granted only after review of individual cases by the regional review board. These patients are usually given a MELD score of 22 (28 in primary hyperoxaluria), with a 10% upgrade every 3 months until they receive a transplant.
Due to the current liver allocation system, in general, LDLT in the USA is offered for the indications listed in Table 1. Included are cirrhotic patients with low MELD scores with complications of end-stage liver disease such as ascites or hepatic encephalopathy, as well as patients with HCC that do not meet the current tumor criteria for DDLT but have shown favorable tumor biology with an adequate time of observation. Also, in regions where the wait time for patients with HCC often exceeds 12 months, LDLT may have a role to reduce drop off from the wait list.
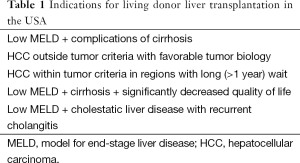
Full table
Patients with HCC outside current tumor criteria are often denied liver transplantation in the USA. Most regions use the Milan criteria, which allow transplantation in patients who have one tumor up to 5 cm in diameter and three tumors up to 3 cm (16). Region 4 (Texas-Oklahoma) uses more liberal T3 tumor criteria (one tumor up to 6 cm, three tumors up to 5 cm, with the maximum total tumor diameter of 9 cm) (17,18). If the patients do not meet their region’s tumor criteria, they either undergo downstaging by locoregional therapy to fit the criteria or are offered LDLT after an observational period to assess tumor biology. Listing on the DDLT list after downstaging is possible only after approval by the regional review board.
Statistics
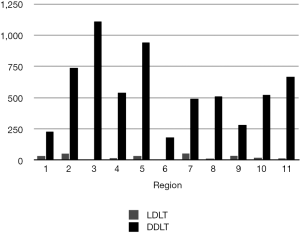
In 2013, 6,455 liver transplants were performed in the USA, 6,203 (96%) from deceased donors and 252 (4%) from live donors (19). The number of DDLT and LDLT performed in each region differs, but across the board LDLT constitutes a small proportion of the overall transplant volume (Figure 3). Further, not all centers offer LDLT—and those that do offer it sometimes have limited experience. In 2013, among the 166 liver transplant centers in the USA, LDLT was performed by 43 centers, with only 8 centers performing 10 or more LDLT (Table 2). None of the centers exceeded 20 LDLT that year.
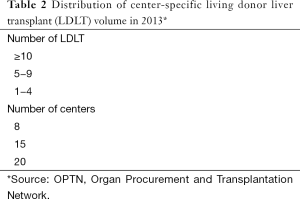
Full table
Figure 1 illustrates the numbers of LDLT and DDLT. In spite of efforts to increase donation rates and increase the use of expanded-criteria liver donors, the total number of deceased donors has been stagnant. The current liver allocation system favors sick patients with high MELD scores and those who receive MELD exception points (e.g., for HCC), but it misses patients with low MELD scores and the significant complications of cirrhosis.
In the meantime, there are approximately 15,000 patients on the waiting list for liver transplantation at any given time. Specifically, in 2014, 10,628 patients were added to the liver transplant waiting list, and 10,603 were removed from the list. Of these, 5,742 (54%) received DDLT and 251 received LDLT. A total of 2,838 (27%) were removed from the list because of death: 1,375 patients died on the waiting list, 1,427 were too sick for transplant, and 26 died during transplant. Given that approximately 15,000 patients are on the waiting list for liver transplant at any given time, the waiting list mortality rate is nearly 20% (19).
Outcomes of LDLT
The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) group showed that LDLT provides survival benefits compared to both staying on the wait list without a liver transplant and receiving DDLT (Figure 4) (20). A2ALL is a consortium of nine centers created to conduct a retrospective and prospective study of the outcomes of donors and recipients after adult LDLT in the USA from 1998 to 2008 (21). The subsequent A2ALL study found that LDLT afforded survival benefits for non-HCC patients with a MELD score ≤15 or >15. However, in patients with HCC, the survival benefit was limited to patients with a MELD score >15 (22).
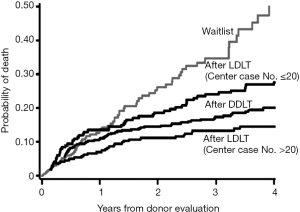
To determine the impact of LDLT on recipients with hepatitis C, a study comparing LDLT and DDLT found that there was no difference in graft survival for hepatitis C patients regardless of the donor type as long as LDLT was performed at high-volume centers. Graft survival after LDLT was worse than that after DDLT when the first 20 LDLT cases were analyzed; however, there was no difference in graft survival between LDLT and DDLT when the center had performed more than 20 LDLT (23).
National data from the Scientific Registry of Transplant Recipients database highlighted that LDLT may be superior to DDLT when performed at experienced centers (defined as centers that had performed >15 LDLT). The 3-year unadjusted graft survival was higher in the LDLT recipients (78.9%) than in the DDLT recipients (77.7%) (P<0.001) at these centers. In addition, recipients with autoimmune hepatitis and cholestatic liver disease had a significantly lower risk of graft failure (24).
Many centers with LDLT offer LDLT to HCC patients who do not meet tumor criteria after assessment of tumor biology, based on the rationale that tumor number and size are not the only indicators of tumor biology and LDLT does not place non-HCC patients who are on the deceased donor wait list at a disadvantage. The most recent A2ALL study found that LDLT was associated with worse oncologic outcomes compared with DDLT, as demonstrated by a higher 5-year recurrence rate of 38% vs. 11% (P=0.0004) (25). However, the outcome is thought to be due to patient selection—in that patients with more advanced tumors were offered LDLT—rather than to the LDLT procedure itself. LDLT patients had higher alpha-fetoprotein levels and a higher tumor burden (larger tumors and a higher rate of patients exceeding Milan and University of California San Francisco tumor criteria), which implies worse tumor biology. This reflects the pattern of practice in that the patients who may not be candidates for DDLT listing are offered LDLT.
The higher recurrence rates of HCC after LDLT observed in the A2ALL study were corroborated by a South Korean study which reported higher recurrence rates in small LDLT grafts (26). However, these findings were not found in other studies that have compared LDLT and DDLT programs. A study from Toronto, Canada, showed that there was no difference between the HCC recurrence rates after LDLT (14.8%) and DDLT (17.0%) (P=0.54) (27). The patients who received LDLT and DDLT were subject to the same tumor criteria. Similar results were seen in the French study by Bhangui et al. (28). Ninomiya et al. also reported similar findings (29). These findings suggest that LDLT itself does not result in worse oncologic outcomes and that the apparent increase in recurrence rate seen in the A2ALL group’s study is due to patient selection. Therefore, the controversy for offering LDLT for HCC patients outside the region’s criteria is based on an ethical issue rather than an oncologic issue. It is difficult to know what posttransplant survival and HCC recurrence rates justify putting a living donor through the risks of the operation. Assessment of tumor biology with locoregional therapy and an observational period is imperative to ensure good posttransplant outcomes in such patients.
Biliary complications
It is well known that biliary complication rates are higher after LDLT than after DDLT (30-32). Although multiple factors may be involved, the key factor is interruption of the bile duct blood supply to the donor and the recipient ducts due to high hilar dissection. Studies from large-volume LDLT centers have suggested that meticulous technique to preserve the blood supply to the donor bile ducts (33-37) and the recipient bile ducts (37-40) are the key steps in reducing biliary complications. The largest direct comparison study performed in the USA found that the biliary complication rate was higher after LDLT than after DDLT (40% vs. 25%; P<0.001). LDLT was more likely to be associated with a higher number of biliary anastomoses (P<0.001) and a higher proportion of patients undergoing Roux-en-Y reconstruction (P<0.001). However, the average number of biliary tract procedures was similar in DDLT and LDLT patients, as was the amount of time between the onset of complications and resolution (41).
Donor safety
Donor hepatectomy is a major operation with potential for major morbidity and mortality. Along with its benefits for the recipient, it carries with it small but real risks for the donor. The largest living liver donor study in the USA was performed by the A2ALL group. Of the 760 donors analyzed, the no-go rate for donation was 2.6%, usually due to unexpected findings in the operating room. The overall donor mortality rate after donation was 0.4%, and the overall complication rate was 40%, with multiple complications occurring in 19% of the patients. The rate of serious complications resulting in lasting disability was 1.1%, with liver failure or death in 0.4% (42). The most common complications were bile leaks (9%), bacterial infections (12%), and incisional hernia (6%). Of note, left-lobe donation was associated with a higher risk of complications on univariate analysis [hazard ratio (HR) =1.60; 95% confidence interval (CI), 0.99−2.56; P=0.05]. When analyzed by a multivariate model, there was a trend towards a higher risk of complications with left-lobe donation (HR =1.55; 95% CI, 0.96−2.51; P=0.08). It is difficult to interpret the data since the overall number of left-lobe donors was small (n=33), which precluded an extensive analysis. The same study also found that blood loss was a predictor of postoperative complications, specifically bile leaks and infections (42).
The rate of donor complications reported in the A2ALL series appear to be slightly higher than what is reported in the single center series from Asia. The reported rate of major complications by Hwang et al. was 1.3% to 6.7% (43) and 4% by Liu and colleagues (44). Potential reasons for this difference are twofold. The impressive low rates of major complications reported by the Asian centers are from high volume single center series where there is less heterogeneity in the operations and the patient care. The A2ALL data is composed by outcomes from multiple centers, each performing relatively low annual volume of liver donor operations. However, the head to head comparisons between the Asian and North American studies may be difficult due to the difference in patient population.
The initial laboratory abnormalities seen after donor hepatectomy normalize within the first few months. However, about 10% of donors can experience platelet counts <150×1,000/mm3 (45), and persistent thrombocytopenia at 1 year was associated with splenomegaly (46). This is likely due to the relative portal hypertension after hepatectomy, where a smaller hepatic mass is exposed to the same amount of portal venous flow. This view is supported by an increase in the spleen volume after donor hepatectomy (46). The long-term effects of these findings are unknown.
Aside from the physiologic sequelae, the quality of life of the donor after donation is favorable. A quality of life survey revealed that donors experience above-average quality of life compared to the general population. However, not surprisingly, the recipient’s death up to 2 years prior to the survey was associated with a poor mental component summary [odds ratio 2.879 (1.391−5.956), P=0.004] (47).
Although it is uncommon, death after donor hepatectomy is worth further discussion. The estimated donor death risk is <1%, as reported by the A2ALL group. The national rate may be difficult to ascertain accurately since there is no national database to register donor deaths beyond early deaths and liver failure. A recent study by Muzaale et al. helped clarify this issue (48). This study included 4,111 living liver donors over the 17-year period reported to the Organ Procurement and Transplantation Network. The Social Security numbers of the donors were acquired and linked to the Social Security Death Master File. Death information was then confirmed with the transplant center. The early death rate was 1.7 deaths per 1,000 donors. The type of graft had no effect on the risk of early death. The rate of catastrophic events was 2.9 per 1,000 donors. Five donors experienced acute liver failure. Three of these donors were salvaged by DDLT, one died, and one improved (48). Of note, all five of these patients had donated right lobes. The long-term mortality rate of 1.2% at 11 years after liver donation was very similar to the rate for matched living kidney donors (1.2% at 11 years), as well as for matched participants in the Third National Health and Nutrition Examination Survey (used as controls) (1.4% at 11 years). The data from this study were obtained and verified by more than one source, and the figures from this study are probably the most accurate estimates of donor mortality at the national level.
Although the risk of death after liver donation is low, it is imperative that the transplant team reduces donor risk as much as possible. Favoring left-lobe grafts over right-lobe grafts has been suggested as one way to potentially reduce donor risk. This trend has been reflected in the gradual change in the pattern of practice. In the A2ALL study, most of the grafts (95%) were right lobes. In the initial study period, the left lobes comprised 2% of grants, and in the more recent era, this rate increased to 7%. The review of the Scientific Registry of Transplant Recipients database also suggested an increase in the annual use of left-lobe donors in the more recent era [2004−2010] compared with the earlier experience [1998−2003], where the rate of left-lobe donors increased from 2.3% to 7.2% (49).
Use of the left lobe is based on the assumption that harvesting a smaller volume of the liver graft from the donor will reduce donor morbidity and improve safety. This appeared to be the case in a large series from Asia. A multicenter study from Japan of 1,680 live donors documented morbidity rates of 8.2% for left lateral section donors, 12% for left-lobe donors, and 19% for right-lobe donors (50). In contrast, some studies have not found a correlation between the type of hepatectomy and the risk of donor death (51,52), and the morbidity with right-lobe donation may decrease with a center’s experience (53). However, left hepatectomy for the donor removes less liver volume and has potential advantages in avoiding postoperative liver failure. Provided that it gives adequate volume for the recipient, it should be preferred (54,55). A study from the USA documented the feasibility of left-lobe LDLT in 21 patients, 16 of whom had portocaval shunts (54). One patient in this study developed small-for-size syndrome, and the 1-year graft survival was 81%. A position paper in 2013 suggested favoring left-lobe grafts over the right-lobe grafts to protect the donors (55).
Conclusions
In the USA, living donors continue to be a small but important source of grafts to address the shortage of liver donors. The current liver allocation system, based on MELD, allows a unique place for LDLT in the system. There has been a gradual trend toward using left lobes over right lobes to reduce donor risk. This appears to be a reasonable option, provided that an adequate graft volume can be provided for the recipient. There are multiple challenges to the growth of LDLT in the USA. These include low center volumes, lack of formal training programs for LDLT, and federal scrutiny of the transplant programs, which may result in rendering the programs risk averse. As we approach the third decade of adult LDLT in the USA, continued vigilance in donor safety and optimal recipient outcomes are required to ensure the growth of LDLT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Strong RW, Lynch SV, Ong TH, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990;322:1505-7. [PubMed]
- Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg 1991;214:428-37; discussion 37-9. [PubMed]
- Hashikura Y, Makuuchi M, Kawasaki S, et al. Successful living-related partial liver transplantation to an adult patient. Lancet 1994;343:1233-4. [PubMed]
- Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg 1996;224:544-52; discussion 552-4. [PubMed]
- Ben-Haim M, Emre S, Fishbein TM, et al. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl 2001;7:948-53. [PubMed]
- Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation 1998;66:1313-6. [PubMed]
- Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl 2000;6:296-301. [PubMed]
- Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg 2001;234:301-11; discussion 11-2. [PubMed]
- Brown RS Jr, Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med 2003;348:818-25. [PubMed]
- Miller C, Florman S, Kim-Schluger L, et al. Fulminant and fatal gas gangrene of the stomach in a healthy live liver donor. Liver Transpl 2004;10:1315-9. [PubMed]
- Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl 2003;9:651-63. [PubMed]
- Amin MG, Wolf MP, TenBrook JA Jr, et al. Expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: a decision analysis. Liver Transpl 2004;10:1468-75. [PubMed]
- Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl 2010;16:874-84. [PubMed]
- Khapra AP, Agarwal K, Fiel MI, et al. Impact of donor age on survival and fibrosis progression in patients with hepatitis C undergoing liver transplantation using HCV+ allografts. Liver Transpl 2006;12:1496-503. [PubMed]
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91-6. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Guiteau JJ, Cotton RT, Washburn WK, et al. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am J Transplant 2010;10:2092-8. [PubMed]
- Onaca N, Davis GL, Goldstein RM, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl 2007;13:391-9. [PubMed]
- Organ Procurement and Transplantation Network. Available online: http://optn.transplant.hrsa.gov
- Berg CL, Gillespie BW, Merion RM, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology 2007;133:1806-13. [PubMed]
- Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg 2005;242:314-23, discussion 323-5. [PubMed]
- Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology 2011;54:1313-21. [PubMed]
- Terrault NA, Shiffman ML, Lok AS, et al. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl 2007;13:122-9. [PubMed]
- Goldberg DS, French B, Abt PL, et al. Superior survival using living donors and donor-recipient matching using a novel living donor risk index. Hepatology 2014;60:1717-26. [PubMed]
- Kulik LM, Fisher RA, Rodrigo DR, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant 2012;12:2997-3007. [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014;97:71-7. [PubMed]
- Sandhu L, Sandroussi C, Guba M, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl 2012;18:315-22. [PubMed]
- Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology 2011;53:1570-9. [PubMed]
- Ninomiya M, Shirabe K, Facciuto ME, et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am Coll Surg 2015;220:297-304.e3.
- Shah SA, Grant DR, McGilvray ID, et al. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transplant 2007;7:161-7. [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg 2006;243:404-10. [PubMed]
- Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant 2008;8:2569-79. [PubMed]
- Soin AS, Kumaran V, Rastogi AN, et al. Evolution of a reliable biliary reconstructive technique in 400 consecutive living donor liver transplants. J Am Coll Surg 2010;211:24-32. [PubMed]
- Takatsuki M, Eguchi S, Tokai H, et al. A secured technique for bile duct division during living donor right hepatectomy. Liver Transpl 2006;12:1435-6. [PubMed]
- Kim SH, Kim YK. Living donor right hepatectomy using the hanging maneuver by Glisson's approach under the upper midline incision. World J Surg 2012;36:401-6. [PubMed]
- Yamamoto S, Sato Y, Oya H, et al. Risk factors and prevention of biliary anastomotic complications in adult living donor liver transplantation. World J Gastroenterol 2007;13:4236-41. [PubMed]
- Ikegami T, Shirabe K, Morita K, et al. Minimal hilar dissection prevents biliary anastomotic stricture after living donor liver transplantation. Transplantation 2011;92:1147-51. [PubMed]
- Lee KW, Joh JW, Kim SJ, et al. High hilar dissection: new technique to reduce biliary complication in living donor liver transplantation. Liver Transpl 2004;10:1158-62. [PubMed]
- Soejima Y, Fukuhara T, Morita K, et al. A simple hilar dissection technique preserving maximum blood supply to the bile duct in living donor liver transplantation. Transplantation 2008;86:1468-9. [PubMed]
- Kim SH, Lee KW, Kim YK, et al. Tailored telescopic reconstruction of the bile duct in living donor liver transplantation. Liver Transpl 2010;16:1069-74. [PubMed]
- Zimmerman MA, Baker T, Goodrich NP, et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl 2013;19:259-67. [PubMed]
- Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant 2012;12:1208-17. [PubMed]
- Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-7. [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Safety of donor right hepatectomy without abdominal drainage: a prospective evaluation in 100 consecutive liver donors. Liver Transpl 2005;11:314-9. [PubMed]
- Trotter JF, Gillespie BW, Terrault NA, et al. Laboratory test results after living liver donation in the adult-to-adult living donor liver transplantation cohort study. Liver Transpl 2011;17:409-17. [PubMed]
- Emond JC, Fisher RA, Everson G, et al. Changes in liver and spleen volumes after living liver donation: a report from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL). Liver Transpl 2015;21:151-61. [PubMed]
- Ladner DP, Dew MA, Forney S, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL). J Hepatol 2015;62:346-53. [PubMed]
- Muzaale AD, Dagher NN, Montgomery RA, et al. Estimates of early death, acute liver failure, and long-term mortality among live liver donors. Gastroenterology 2012;142:273-80. [PubMed]
- Saidi RF, Jabbour N, Li Y, et al. Is left lobe adult-to-adult living donor liver transplantation ready for widespread use? The US experience (1998-2010). HPB (Oxford) 2012;14:455-60. [PubMed]
- Umeshita K, Fujiwara K, Kiyosawa K, et al. Operative morbidity of living liver donors in Japan. Lancet 2003;362:687-90. [PubMed]
- Cheah YL, Simpson MA, Pomposelli JJ, et al. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver Transpl 2013;19:499-506. [PubMed]
- Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology 2008;135:468-76. [PubMed]
- Uchiyama H, Shirabe K, Nakagawara H, et al. Revisiting the safety of living liver donors by reassessing 441 donor hepatectomies: is a larger hepatectomy complication-prone? Am J Transplant 2014;14:367-74. [PubMed]
- Botha JF, Langnas AN, Campos BD, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl 2010;16:649-57. [PubMed]
- Roll GR, Parekh JR, Parker WF, et al. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver Transpl 2013;19:472-81. [PubMed]


