Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with Child B or C cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is often associated with liver cirrhosis (1,2). Most cases of HCC are attributable to chronic liver disease related to hepatitis viruses, including hepatitis B virus or hepatitis C virus. Because laparoscopic liver resection (LLR) is reported to be associated with improved outcomes, LLR can be a useful first-line treatment for HCC (3,4). Compared with open liver resection, LLR is reported to have the advantages of less blood loss, fewer blood transfusions, lower morbidity, and shorter hospital stays. The overall survival (OS) and disease-free survival (DFS) outcomes are reportedly similar for LLR and open liver resection, although further studies are required to evaluate the impact of LLR on long-term results (4).
Most studies consider Child B or C grade cirrhosis a contraindication for liver resection, and surgeons face a considerable challenge in dealing with non-compensated cirrhotic patients. However, because LLR for the treatment of HCC has the advantage of minimal stress to the patient, favorable outcomes should be expected after LLR in patients with liver cirrhosis (5-7). Although good outcomes in these patients have been reported, severe cirrhosis was excluded in those studies (5-10). There have also been few reports of the oncological outcomes of patients with Child B or C cirrhosis (11).
Therefore, in this study, we retrospectively analyzed the clinical and oncological outcomes after LLR in patients with Child B or C cirrhosis.
Methods
Between January 2004 and December 2013, LLR was performed in 232 patients with HCC at the Department of Surgery, Seoul National University Bundang Hospital. Among these patients, 141 had pathologically proven associated liver cirrhosis. Sixteen patients classified with Child B or C cirrhosis were included in this study. The clinical data of the patients were analyzed retrospectively by reviewing their medical records and radiological images. Written informed consent was obtained from all patients before surgery, and this study was approved by the Institutional Ethical Committee at our hospital. The selection of the surgery type was based on the functional liver reserve and the location of the lesion. Anatomical liver resection was preferred when the lesion was deeply located and the remaining liver function was expected to be adequate, whereas non-anatomical liver resection was performed when the tumor was peripherally located or anatomical liver resection was not feasible.
Preoperative evaluation
To assess the functional liver reserve, all patients were preoperatively evaluated with liver function tests, the indocyanine green retention rate at 15 min (ICG-R15), and the Child-Pugh classification. The preoperative radiological examinations used were triphasic computed tomography (CT) or contrast-enhanced magnetic resonance imaging.
Operative technique
The operative techniques for LLR at our hospital have been described previously (12). A pneumoperitoneum was established through a 10-mm umbilical port, and this was maintained at 12 mmHg. An infraumbilical incision was used in cirrhotic patients to avoid injury to a recannulated umbilical vein. If the patient has a lesion in segment 7 or 8 of the liver, we use a port inserted through the intercostal space (13). Laparoscopic ultrasonography was used for the precise localization of the tumor, to identify any satellite nodules, and to obtain an adequate tumor-free margin. Patient positioning, trocar placement, and the type of resection used were carefully selected according to tumor location. The superficial hepatic parenchyma was transected using a harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA), and the deeper portion of the parenchyma was transected using a laparoscopic cavitron ultrasonic surgical aspirator (Valleylab, Inc., Boulder, CO, USA). Bleeding from small branches of the hepatic veins was controlled with endoclips and a sealing device. Pringle maneuver was used in some cases for non-anatomical resections. After careful hemostasis, a fibrin glue sealant (Greenplast, Green Cross, Seoul, Korea) or polyethylene glycolic acid (Neoveil, Gunze, Tokyo, Japan) was applied routinely to the cut surface. The resected specimen was inserted into a protective bag and retrieved through the epigastric or subumbilical port site or a suprapubic incision. After the surgical field was irrigated, a silastic drain was inserted routinely, and the wound was closed in layers.
Postoperative care and follow-up
Postoperatively, all patients underwent daily follow up of liver functions and abdominal CT was done routinely on fifth postoperative day. If there is no abdominal collection, and drainage of clear serous fluid less than 100 cc every 24 hours with no bile content and negative bacteriological cultures, we remove the abdominal drain.
Follow-up CT was performed within three months after the procedure in all patients, to evaluate the effectiveness of the treatment. All patients were followed-up thereafter with CT imaging, serum biochemical tests, and a clinical examination every 3 months for the first two years and every six months afterwards.
Clinical outcomes
Postoperative morbidity was defined as events occurring during the first 60 PODs and was graded according to the Clavien-Dindo classification (14). Postoperative liver cell failure was defined according to the International Study Group of Liver Surgery (ILSGLS) (15).
OS is calculated from the day of surgery to the day of death or last follow up. DFS is calculated from the day of surgery to the day of tumor recurrence or the day of death or last follow up.
Statistical analysis
Data are expressed as medians (ranges) or means ± standard deviations. The survival curves were estimated with the Kaplan-Meier method. All statistical analyses were performed with IBM-SPSS version 20 for Windows. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
There were ten (62.5%) men and six (37.5%) women in the study population, with a median age of 59.5 years (range, 44-83 years). Among these patients, 13 (81.3%) had Child B cirrhosis and three (18.8%) had Child C cirrhosis. In terms of etiology, 13 (81.3%) patients had hepatitis B, two (12.5%) patients had hepatitis C, and one (6.3%) patient had both hepatitis B and C.
The median serum albumin was 3.1 g/dL (range, 2.7-3.4 g/dL), bilirubin 2.1 mg/dL (range, 0.9-3.8 mg/dL), international normalized ratio 1.36 (1.05-1.9), alanine amino-transferase 51.5 IU/L (range, 25-308 IU/L), and aspartate amino-transferase 53 IU/L (range, 14-684 IU/L). The median preoperative platelets count was 82×103/mcL [(24-141)×103/mcL], alpha feto-protein 36.5 ng/mL (range, 2.1-3,500 ng/mL), and ICG-R15 11.9% (4.8-54.4%).
LLR procedures included: tumorectomy (n=10), segmentectomy (n=2), left lateral sectionectomy (n=3), or right anterior sectionectomy (n=1). The tumors were located in the left lobe in six patients (37.5%), in the right lobe in nine patients (56.3%), and in both lobes in one patient (6.3%). The median size of the tumor was 2.4 cm (range, 1.0-4.5 cm). Ascites was present in five patients (31.3%). None of them received preoperative diuretic therapy. Previous trans-catheter arterial chemo-embolization (TACE) had been performed in eight (50%) patients and previous radiofrequency ablation (RFA) had been performed in one (6.3%) patient.
Intraoperative and postoperative clinical outcomes
The median operation time was 215 min (range, 75-540 min), the median estimated blood loss was 350 mL (range, 100-1,000 mL), and five patients (31.3%) received blood transfusion. The Pringle maneuver was used in three patients with a median duration of 20 min (range, 10-60 min). The median postoperative hospital stay was 8 days (range, 4-21 days).
The median resection margin was 0.7 cm (range, 0.1-2.0 cm). There were no intraoperative mortalities, early mortalities, or reoperations. Clavien-Dindo grade I complications were recorded in one patient and grade IIIa complications in two patients. The patient with Clavien-Dindo grade I complications had a transient liver function abnormality, which recovered spontaneously without any specific medical therapy apart from the use of diuretics. The Clavien-Dindo grade IIIa complications were pleural effusion in one (6.3%) patient who had been treated with ultrasonography-guided tube drainage; and variceal bleeding in one (6.3%) patient, which was managed with endoscopic banding. These two patients had combined transient liver failure, which recovered spontaneously without any specific medical therapy apart from the use of diuretics. There was no intractable postoperative ascites. No complications were related to the use of intercostal trocars.
Survival and recurrence
The mean follow-up period was 51.6±34.6 months. HCC recurred in eight (50%) patients: intrahepatic in seven (43.8%) patients and extrahepatic (brain metastasis) in one (6.3%) patient. The treatments for recurrence were reoperation in one (6.3%) patient, TACE in one (6.3%) patient, RFA in one (6.3%) patient, and combined TACE and RFA in four (25%) patients. One patient (6.3%) received tumorectomy for HCC recurrence, which was approached laparoscopically, but open conversion occurred due to adhesions.
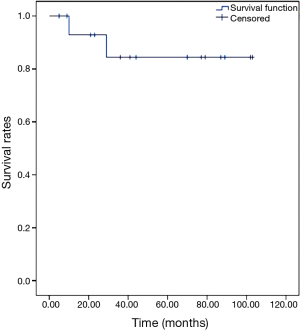
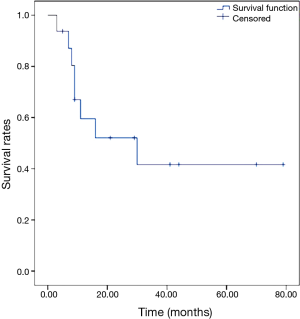
The 5-year OS rate was 84.4%, as shown in Figure 1. The 5-year DFS rate was 41.7%, as shown in Figure 2.
Discussion
Liver resection is the principal treatment for HCC (2,6,7). Because patients with HCC usually have associated chronic liver disease or cirrhosis, these patients may be predisposed to hepatic failure after surgery. Therefore, it is important to preoperatively evaluate the future liver remnant and liver function before selecting liver resection. Liver resection is usually contraindicated in patients with cirrhosis, especially when they have Child B or Child C cirrhosis (6,7). For these patients, liver transplantation may be the best choice in terms of eradicating the malignancy and restoring liver function. However, with the shortage of donor livers, the high cost, and the life-long requirement for immunosuppressive treatment, liver transplantation is not available to most patients.
Recently, LLR has been introduced as an appropriate treatment for HCC (8-10). This minimally invasive surgery may have a role in the treatment of patients with clinically significant cirrhosis (8-10,16-18). Many studies have shown that LLR for HCC is superior to open liver resection in terms of the perioperative results, especially in cirrhotic patients (17-19).
Postoperative ascites is reportedly reduced when laparoscopy is used. LLR minimizes the interruption of the porto-systemic collateral vessels because the incisions in the anterior abdominal wall are small. Less operative trauma and less injury to the collateral vessels may reduce the rate of liver failure and the recurrence of ascites after LLR in patients with severe cirrhosis. Our study shows that LLR is safe for patients with Child B and C cirrhosis, causing no significant postoperative complications, such as intractable ascites.
The incidence of liver failure is also reported to be lower after LLR than after open liver resection (8,10,16). In our study, transient liver failure occurred in three patients (18.8%), but recovered spontaneously. Another advantage of LLR is the shorter hospital stay (3,6,9,16). These outcomes demonstrate that LLR is a relatively safe procedure in severely cirrhotic patients.
It was initially feared that LLR would reduce the surgical margin because palpation is not possible. Manual exploration of the cirrhotic liver can even be difficult during open surgery, because the liver is hard and nodular, and palpation is probably less important when an anatomical resection is planned. However, haptic feedback transmitted through the laparoscopic instruments is possible. Also, intraoperative ultrasound should be systematically used to locate the tumor, making it possible to maintain the intended margin.
In this study, blood loss and the rate of transfusion were similar to those in other reports (3-5,9,17,20). To reduce blood loss during LLR, it is important to use meticulous technique during parenchymal transection. The Pringle maneuver can be applied during LLR to reduce blood loss (3-6). Some researchers also advocate increasing the pneumoperitoneal pressure to minimize bleeding during surgery (6-8). Precoagulation techniques using microwave coagulation or RFA can also be useful in reducing blood loss in cirrhotic patients (4).
In this study, the postoperative outcomes of these severely cirrhotic patients were similar to the outcomes reported in other studies (4,6,7,20,21). Based on the results of the present study, we infer that the use of LLR does not compromise oncological principles in patients with HCC and clinically significant cirrhosis. The present 5-year OS and DFS rates were similar to those in other studies (5,9,20).
In conclusion, this study shows that LLR is feasible and safe in patients with Child B or C cirrhosis. Further research is required to clarify the role of LLR in these patients, including prospective, randomized controlled trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Written informed consent was obtained from all patients before surgery, and this study was approved by the Institutional Ethical Committee of Seoul National University Bundang Hospital.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Mikhail S, Cosgrove D, Zeidan A. Hepatocellular carcinoma: systemic therapies and future perspectives. Expert Rev Anticancer Ther 2014;14:1205-18. [PubMed]
- Fancellu A, Rosman AS, Sanna V, et al. Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res 2011;171:e33-45. [PubMed]
- Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23. [PubMed]
- Piardi T, Sommacale D, Baumert T, et al. Laparoscopic resection for hepatocellular carcinoma: comparison between Middle Eastern and Western experience. Hepatobiliary Surg Nutr 2014;3:60-72. [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-81. [PubMed]
- Truant S, Bouras AF, Hebbar M, et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 2011;25:3668-77. [PubMed]
- Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol 2014;20:4892-9. [PubMed]
- Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 2009;23:1807-11. [PubMed]
- Memeo R, de’Angelis N, Compagnon P, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg 2014;38:2919-26. [PubMed]
- Abdel-Atty MY, Farges O, Jagot P, et al. Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg 1999;86:1397-400. [PubMed]
- Han HS, Cho JY, Yoon YS. Techniques for performing laparoscopic liver resection in various hepatic locations. J Hepatobiliary Pancreat Surg 2009;16:427-32. [PubMed]
- Lee W, Han HS, Yoon YS, et al. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci 2014;21:E65-8. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [PubMed]
- Herman P, Coelho FF. Laparoscopic resection for hepatocellular carcinoma: eastern and western experiences. Chin J Cancer Res 2014;26:234-6. [PubMed]
- Chen PD, Wu CY, Wu YM. Expanding the selection criteria of laparoscopic hepatectomy for hepatocellular carcinoma. Chin J Cancer Res 2014;26:360-1. [PubMed]
- Franssen B, Alshebeeb K, Tabrizian P, et al. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg 2014;260:650-6; discussion 656-8. [PubMed]
- Chau GY. Resection of hepatitis B virus-related hepatocellular carcinoma: evolving strategies and emerging therapies to improve outcome. World J Gastroenterol 2014;20:12473-84. [PubMed]
- Xiong JJ, Altaf K, Javed MA, et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol 2012;18:6657-68. [PubMed]
- Cannon RM, Saggi B, Buell JF. Evaluation of a laparoscopic liver resection in the setting of cirrhosis. HPB (Oxford) 2014;16:164-9. [PubMed]