
Adjuvant chemotherapy for intrahepatic cholangiocarcinoma: approaching clinical practice consensus?
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a rare and aggressive primary liver malignancy. In the United States, the incidence of ICC is estimated at 1.2 cases per 100,000 person years (1). Surgical resection for ICC represents the only curative option; however, ICC is frequently diagnosed at advanced stages, precluding resection. Historically survival in ICC has been poor. Even with curative intent surgical resection, median survival is 28 months and 5-year overall survival is 30% (2).
Due to the rarity of the disease, there is minimal level one evidence to guide clinical management of patients with ICC and there is no clinical consensus on the appropriate use of neoadjuvant and adjuvant treatment. There is conflicting data from randomized controlled trials as to the benefit of adjuvant chemotherapy in resected biliary tract cancers (3-6). While it is known that positive lymph nodes (LN), advanced T stage and positive surgical resection margins are poor prognostic factors (2,7-12), a recent meta-analysis found no additional benefit to adjuvant chemotherapy or radiotherapy in patients undergoing surgical resection (2). However, some subgroup analyses have suggested that patients with advanced T stages or large tumors, positive margins and/or positive LNs, may derive a survival benefit from adjuvant chemotherapy (13-15). In order, to further evaluate the utilization and role of adjuvant chemotherapy for resected ICC in current clinical practice, a large population-based data set was analyzed to characterize chemotherapy utilization in ICC over the last 15 years and to determine whether or not changing practice patterns may have impacted survival. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.06.12/rc).
Methods
An augmented version of the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) program database was used to identify patients diagnosed with ICC from 2000–2014. The SEER database collects patient, tumor and treatment characteristics on patients representing 28% of the United States population (16). The augmented version includes additional chemotherapy data.
Patients age 18 years or older who were diagnosed with ICC from 2000–2014 were identified using the World Health Organizations’ International Classification of Disease, 3rd edition (Table S1). Patients were included if they underwent a therapeutic intent surgical resection for ICC (Table S2). Patients with metastatic disease or those whom received radiation therapy as a component of first-line therapy were excluded. The study was exempt from review by the Institutional Review Board of the University of Minnesota as only de-identified patient data was used.
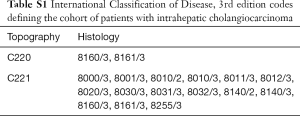
Full table
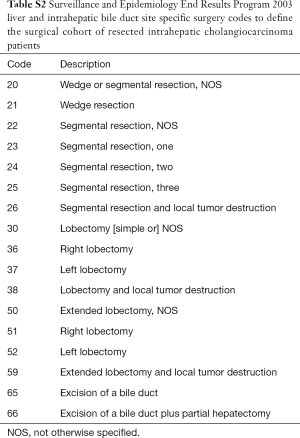
Full table
Patients were stratified into three cohorts based on date of diagnosis, 2000–2004, 2005–2009, and 2010–2014. Patient, tumor, and treatment characteristics as well as survival was analyzed for all three groups. A 2-sided chi-square test and Cochran-Armitage test for trend were used to compare patient characteristics and chemotherapy utilization rates during the 15-year period. Multivariable analysis of factors associated with the receipt of chemotherapy was performed on patients in each time period. Kaplan-Meier survival curves and Cox proportional hazard ratio (HR) models were performed to evaluate overall survival. Statistical significance was considered as P≤0.05. All statistical analysis was performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 1,223 patients underwent surgical resection for ICC from 2000–2014 and met study inclusion criteria. All patients were divided into three cohorts based on time of diagnosis; 194 patients were diagnosed in 2000–2004, 379 patients were diagnosed in 2005–2009 and 650 patients were diagnosed in 2010–2014. Patient, tumor and treatment characteristics for the three cohorts are listed in Table 1.
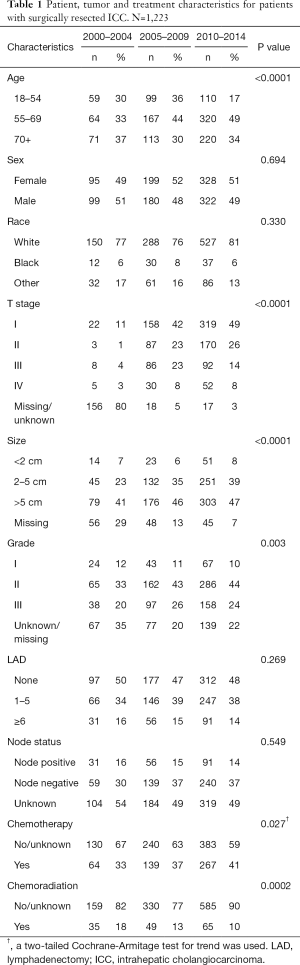
Full table
Utilization of chemotherapy
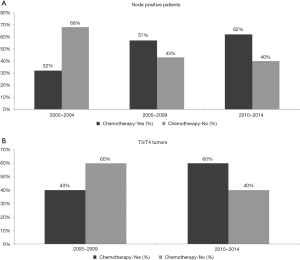
There was a significant trend for increased chemotherapy utilization over the three time periods (33%, 37% and 41% in 2000–2004, 2005–2009 and 2010–2014 respectively, P=0.027). There was an increase in the utilization of chemotherapy in patients with node positive disease from 32% to 57% to 62% (Figure 1A, P=0.014), but not in node negative patients (41% to 36% to 43%, P=0.381, data not shown). The T stage classification was missing in 80% of patients in the 2000–2004 cohort. For this reason, T stage was not included in the analysis for the first time period. From 2005–2009 to 2010–2014 there was a significant increase in chemotherapy use in patients with T3/T4 tumors from 46% to 60% (Figure 1B, P=0.004); however, chemotherapy utilization did not change in those with T1 (33% in 2005–2009 and 34% in 2010–2014, P=0.421) and T2 disease (43% in 2005–2009 and 40% in 2010–2014, P=0.785, data not shown).
Multivariable regression analysis was performed to evaluate factors associated with chemotherapy utilization in each time period (Table 2). All regression models included patient age, gender, race, grade, LN status and T stage. T stage was excluded from the analysis for the first time period [2000–2004] due to missing data, as described above. From 2000–2004, only missing grade compared to grade I was a predictor of decreased odds of chemotherapy. From 2005–2009, LN positive status was independently associated with an increased odds of receipt of chemotherapy. Factors associated with a decreased odds of chemotherapy use were increasing age and male gender. From 2010–2014 both LN positive status and T stage 3/4 were independently associated with an increased odds of chemotherapy receipt. The factors associated with a decreased odds of chemotherapy use were age and male gender.
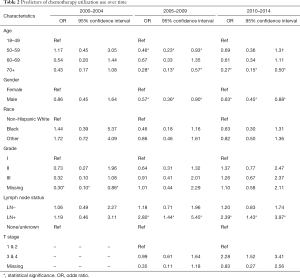
Full table
Overall survival
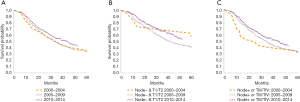
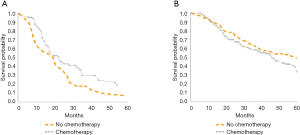
Median overall survival was 32 months during both the early time periods (2000–2004 and 2005–2009) and improved to 41 months in 2010–2014 (P=0.033, Figure 2A). For patients with low-risk tumor characteristics (LN negative disease and T1/T2 tumors), the median survival was 55 months in 2000–2004, 40 months in 2005–2009, and 52 months in 2010–2014 (P=0.045, Figure 2B). For patients with high-risk tumor characteristics (LN positive disease or T3/T4 tumors), median survival was 23.5, 34.5 and 44 months for 2000–2004, 2005–2009, 2010–2014 respectively, (P=0.03, Figure 2C). Among patients with LN positive disease, median overall survival was 19 months when patients received no chemotherapy and 23 months when patients received chemotherapy (Figure 3A, P≤0.02). For patients with LN negative disease, there was no significant improvement in median overall survival associated with receipt of chemotherapy—median overall survival was 46 months without chemotherapy and 59 months with chemotherapy (Figure 3B, P=0.08).
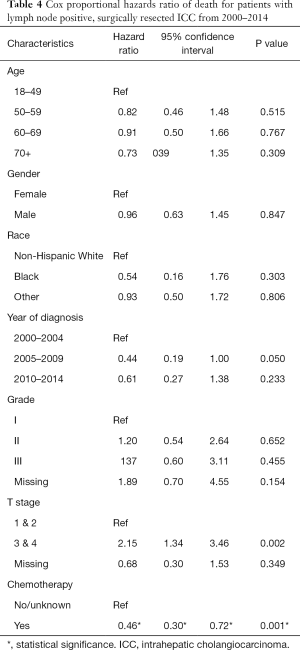
Using cox proportional hazard models for the entire cohort, male sex, advanced grade (grade III compared to grade I), advanced T stage (stage 2 compared to stage 1, stage 3/4 compared to stage 1) and LN positive disease were all associated with an increase in the hazard ratio of death (Table 3). Receipt of chemotherapy was not found to be protective (HR 0.94, P=0.486). A separate analysis was performed on the subgroup of patients with LN positive disease (Table 4). In this patient cohort, only receipt of chemotherapy was found to significantly decrease the hazard ratio of death, while advanced T stage (T3/T4) was found to significantly increase the hazard ratio of death.
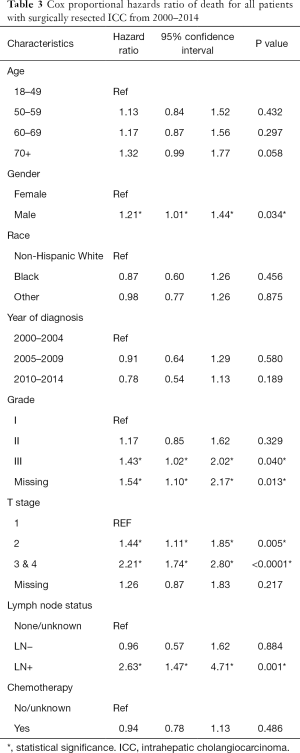
Full table
Full table
Discussion
This study used a national dataset to evaluate the utilization of adjuvant chemotherapy in surgically resected ICC patients over a 15-year period, and found that the use of adjuvant chemotherapy has evolved. The administration of chemotherapy has increased over time, largely due to increased use in patients with LN positive disease and/or T3/T4 tumors. On the other hand, the use of chemotherapy has remained stable in patients with LN negative disease and/or T1/T2 tumors. Furthermore, multivariate analysis of the most recent time period [2010–2014] revealed that LN positive status and T stage 3/4 were independently associated with an increased odds of chemotherapy receipt. Over the 15-year time period, overall survival improved for all patients, from 32 months [2000–2004] to 41 months [2010–2014], and was most dramatic for patients with LN positive disease or T3/T4 tumors. This improvement in survival may, in part, be explained by increased use of chemotherapy in these high-risk patients. In patients with LN positive disease, adjuvant chemotherapy was the only factor that decreased the hazard ratio of death.
ICC has a high rate of recurrence and poor overall survival when treated with surgical resection alone, highlighting the importance of adjuvant therapies. The ABC-02 trial evaluated chemotherapy in advanced biliary tract cancer and found a survival benefit of 3 months for combination gemcitabine and cisplatin over gemcitabine alone (17). The study included patients with advanced biliary tract cancers, less than 60% of whom had cholangiocarcinoma and only 20% of patients were initially treated with curative intent surgery (17). Two recent randomized controlled trials looked at the role of adjuvant chemotherapy specifically. The BILCAP study was intended to more clearly define the role of adjuvant chemotherapy in biliary tract tumors by randomizing patients after upfront curative intent resection to receive either capecitabine or observation alone (6). After a median follow-up of 60 months, there was no significant difference in overall survival when analyzed under an intention-to-treat analysis—though interpretation of the results of the study have sparked much controversy (6,18).The Prodige 12 trial evaluated adjuvant gemcitabine and oxaliplatin and found no significant difference in recurrence free survival between the two arms, with a median follow-up of 46.5 months (5).
Previous studies have attempted to clarify the efficacy and role of chemotherapy in the adjuvant setting by retrospectively analyzing large data sets. Most studies have found that adjuvant chemotherapy was not associated with improved survival when the entire cohort of surgically resected ICC patients was analyzed (2,13). Multiple studies have determined that positive LN status, advanced T stage and positive surgical resection margins are all poor prognostic factors (2,7-12). Additional studies have shown an association between the use of adjuvant chemotherapy and improved survival when administered to the subgroup of patients with poor prognostic factors (13-15,19,20). For example, Miura et al. analyzed 2,751 patients in the National Cancer Database and found that the addition of chemotherapy was associated with a survival benefit only in patients with positive LNs, advanced T stages and positive resection margins (14). This was replicated in a large review of the SEER database (13). Further, a systematic review encompassing over 6,000 patients with biliary tract cancer found no significant improvement in overall survival with adjuvant treatment, except for in patients with high-risk features (positive margins and positive LNs) (21). The current study, redemonstrated that patients with LN positive or T3/T4 disease have worse overall survival, and suggests that the increased utilization of chemotherapy over time in these subgroups of patients may help explain the improvements noted in overall survival.
Despite LN positivity being an important factor to help determine which patients may benefit from chemotherapy as demonstrated in this and prior studies (11,13,14,21,22), the rate of any lymphadenectomy in the SEER database was only 52.1% during the period of study, and did not change over the 15-year time period. Previous studies have reported low rates of pathologic LN evaluation in ICC, with only 10% of patients undergoing an adequate [defined as 6 or more LN evaluated (23)] lymphadenectomy (13,24). Selective, inadequate and omitting lymphadenectomy in patients with ICC may result in nodal under-staging and possibly under treatment with adjuvant therapy.
Due to the sparsity of strong level one evidence, current National Comprehensive Cancer Network guidelines are not specific with respect to adjuvant treatment (25). For patients without evidence of residual disease, either observation, clinical trials or chemotherapy are acceptable options. In patients with microscopic residual disease or positive LNs, recommendations include enrollment in a clinical trial or chemotherapy with or without chemoradiation. Hopefully, the ongoing ACTICCA-1 trial, which initially randomized patients to observation or adjuvant gemcitabine-cisplatin after curative intent resection will help clarify the role of adjuvant chemotherapy (26). However, due to the rarity of biliary tract tumors, randomized trials uniformly include patients with ICC, extrahepatic cholangiocarcinoma, and gallbladders cancer. For instance, the Prodige 12 trial included only 86 patients with ICC while the BILCAP trial included only 84 patients (5,6). Although this improves the study’s statistical power, this practice may not be clinically appropriate, particularly in light of emerging data that genetic mutations differ across these distinct biliary tract cancers (27).
Despite the lack of level one evidence or strong clinical guidelines, clinical practice has evolved towards the use of adjuvant chemotherapy predominantly in patients with poor prognostic features. In 2000–2004 chemotherapy was administered to 33% of patients with resected ICC and increased to 41% in 2010–2014. However, this increase is due to increased utilization in patients with LN positive disease and/or T3/T4 tumors, whereas there is not a corollary increase in patients with node negative or low T stage disease. From 2000–2004, no clinically significant predictors of chemotherapy utilization were identified. In the most recent time period [2010–2014], patients with LN positive disease or T3/T4 tumors had an increased odds of chemotherapy receipt, while older patients and males had a decreased odds of receiving chemotherapy. Median overall survival for all surgically resected patients improved from 32 months in 2000–2004 to 41 months in 2010–2014. In patients with LN positive disease or T3/T4 tumors, median overall survival improved from 23.5 to 44 months while median survival was relatively stagnant among patients with LN negative and T1/T2 disease. The evolution of chemotherapy utilization may be partially responsible for the improvements seen in overall survival.
Although this is a robust, population-based study on chemotherapy in surgically resected ICC, it is important to acknowledge some of the study’s limitations. As a review of a large population dataset, it is subject to retrospective and reporting biases. Furthermore, as a retrospective study it is subject to selection bias as there is no randomization of treatments cohorts. Also, using the SEER database there were a significant number of missing values, particularly for T stage in the early cohorts. The SEER database also does not specify the date of chemotherapy, the specific agents/drugs given, or any details about the duration of therapy. Lastly, SEER does not report resection status or contain information on recurrence status. Resection margin status is an important prognostic indicator in resected ICC and likely factors into adjuvant treatment decisions. Nonetheless, given the rarity of ICC, this is a large study evaluating adjuvant chemotherapy for ICC over-time in the United States.
Despite these limitations, this study suggests that clinicians are administering chemotherapy with increased frequency to patients with poor prognostic features following surgical resection of ICC. Specifically, clinicians are increasing chemotherapy utilization in patients with LN positive disease or T3/T4 tumors, while the utilization of chemotherapy among patients with LN negative disease or T1/T2 tumors has remained stable. Furthermore, the increased utilization of chemotherapy in patients with poor prognostic features may partially explain the improvement in overall survival over the last 15 years. It is likely that the previous retrospective studies have influenced clinical management contributing to this shift, as robust definitive level one evidence is still lacking. The data from this study suggests that the oncologic community is moving towards a clinical practice consensus on the use of adjuvant chemotherapy for ICC, but is undoubtedly awaiting further clarity that can only be provided by well-designed, robust randomized clinical trials.
Acknowledgments
The authors of the manuscript would like to acknowledge Stephanie Lundgren and the Division of Surgical Oncology at the University of Minnesota for assistance in the production of the manuscript.
Funding: The manuscript was in part funded by the Institute of Basic and Applied Research in Surgery at the University of Minnesota, the VFW fund and the University of Minnesota Department of Surgery Cancer Fund.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.06.12/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.06.12/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.06.12/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The study was exempt from review by the Institutional Review Board of the University of Minnesota as only de-identified patient data was used. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J Clin Oncol 2017;35:4006. [Crossref]
- Edeline J, Bonnetain F, Phelip JM, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol 2017;35:225. [Crossref]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 2016;18:79-87. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-54. [Crossref] [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-33. [Crossref] [PubMed]
- Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 2007;11:1488-96; discussion 1496-7. [Crossref] [PubMed]
- Current survival and treatment trends for surgically resected intrahepatic cholangiocarcinoma in the United States. J Gastrointest Oncol 2018;9:942-52. [Crossref] [PubMed]
- Miura JT, Johnston FM, Tsai S, et al. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:3716-23. [Crossref] [PubMed]
- Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:2209-17. [Crossref] [PubMed]
- SEER Incidence Database - SEER Data & Software. 2018. Available online: https://seer.cancer.gov/data/
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Malka D, Edeline J. Adjuvant capecitabine in biliary tract cancer: a standard option? Lancet Oncol 2019;20:606-8. [Crossref] [PubMed]
- McNamara MG, Walter T, Horgan AM, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol 2015;38:382-7. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Kizy S, Altman AM, Marmor S, et al. Surgical resection of lymph node positive intrahepatic cholangiocarcinoma may not improve survival. HPB (Oxford) 2019;21:235-41. [Crossref] [PubMed]
- Amin MB. AJCC cancer staging manual. Eight edition. Chicago IL: American Joint Committee on Cancer, Springer, 2017.
- Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg 2018;22:668-75. [Crossref] [PubMed]
- Benson AB, D'Angelica MI, Abbott DE, et al. NCCN Clinical Practice Guidelines: Hepatobiliary Cancers. NCCN. 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 5/30/2018.
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016;122:3838-47. [Crossref] [PubMed]


