
Is direct acting antiviral therapy for hepatitis c viral infection associated with increased risk of hepatocellular carcinoma before or after liver transplantation?
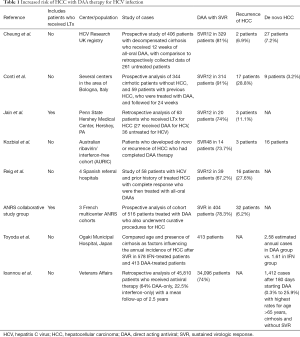
Direct acting antiviral (DAA) therapy revolutionized the curative therapy for >90% of hepatitis C virus (HCV) infected patients. Initially, in late 2016, concerns were raised by several European centers that DAA therapy may be increasing the incidence of hepatocellular carcinoma (HCC) recurrence or de novo HCC (Table 1). Singal et al. (1) compared the frequency of HCC recurrence in patients who received DAA therapy for HCV versus patients who did not receive DAA, among patients with HCV positive patients associated HCC, cured with complete response by various modalities [resection, trans arterial chemo embolization (TACE), local ablation, stereotactic body radiation therapy (SBRT) or other methods]. This was a large multicenter study which included 31 centers from North America and Canada. There were 304 HCV-positive patients treated with DAA therapy after HCC cure and 489 HCV-positive subjects without DAA therapy after curing the HCC. Singal et al. found the total recurrence rate was 42.1% (128 patients) in the treatment group and 58.9% (288 patients) without treatment group (P=0.33), that was statistically not significant (1).
Full table
While this data is essential to demonstrate the recurrence of HCC with DAA therapy, anxiety remains for the group of patients who develop de novo or recurrent HCC following DAA therapy even after liver transplantation (2). However, in the above Singal et al. study, the untreated group included some of the patients claimed to have received DAA therapy after 365 days of complete response to HCC or after various landmark time frames. This makes the control group somewhat unsatisfactory.
In the non-liver transplant patients, a controversy has emerged over if the rate and recurrence of HCC is higher post DAA therapy to treat HCV-induced cirrhosis. Some European centers suggest with DAA therapy higher risk of de novo HCC and recurrence of HCC. Reig et al. reported, from four participating centers from Spain, consisting of 103 patients with HCC who received DAA, 27.6% either had an increased in size of HCC or developed de novo HCC at a median follow up of 5.7 months (3). Another study from Japan by Toyoda et al. included 413 patients after sustained virologic response (SVR) with DAA therapy. They observed a significant increase in the incidence of HCC in patients more than 65 years of age with cirrhosis, and double rate of HCC after the SVR from DAA therapy, on a yearly basis (4). Conti et al. from Italy in 2016 accounted for 344 patients post-DAA therapy (5). Within the six months follow-up period, they observed nine cases of de novo HCC out of 285 cases. Furthermore, 17 patients out of 59 stable HCC treated patients had a rapid recurrence of HCC. Kozbial et al. from the AURIC (Austrian Ribavirin/Interferon-Free Cohort) clinical trial, consisting of HCV positive patients treated with DAA therapy, observed an unexpected increased rate of HCC (6).
However, Cheng et al. from the UK group, in 406 HCV positive patients treated with DAA therapy, did not observe an increased rate of HCC post-DAA therapy (7). Similarly, a French multicenter ANRS group also did not observe increased risk of HCC in over 6,000 patients post DAA therapy (8). Recently, Ioannou et al. have suggested a model for estimating the risk of HCC after antiviral treatment for HCV infection. They studied 45,810 patients from Veterans affairs and developed a model to assess the risk of HCC post DAA therapy. Authors describe a group of patients >65 years of age, with cirrhosis, who did not achieve SVR, with an unusually elevated three-year risk of HCC of up to 26% (9). This is somewhat similar to what we have observed in post-liver transplant recipients at our institution who did not maintain SVR (2).
A hypothetical possibility is that viral relapse may stimulate an immune or oncogenic environment in the host which could promote HCC recurrence, including after liver replacement with immunosuppression. To answer this question, we would need data from a larger population, involving multiple centers. If this is a real phenomenon, then a major change in the practice of LTx for HCC within Milan criteria may be required. If there is a significant effect on DE Novo or recurrence HCC post-DAA therapy than this potential risk should be discussed with the patients. However, efficacy of DAA therapy to eradicate the virus is so vital for over 90% of HCV positive subjects that a separate approach can be considered. We have suggested a trial where Sorafenib, in a prophylactic dose can be utilized with DAA therapy (10). Furthermore, we have discussed transcriptome signature gene analysis to find early recurrence of HCC after DAA therapy as another approach for this group of patients with cellular biological and immunologic-based experiments (2), A large multicenter prospective trial, specifically designed to identify the risk of De Novo or recurrent HCC with the use of DAA therapy, in transplant or non-transplant subjects, with SVR or without SVR, is required to answer this vital question.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019;156:1683-92.e1. [Crossref] [PubMed]
- Jain A, Miller D, Schreibman I, et al. Is there increased risk of hepatocellular carcinoma recurrence in liver transplant patients with direct-acting antiviral therapy? Hepatol Int 2019;13:190-8. [Crossref] [PubMed]
- Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719-26. [Crossref] [PubMed]
- Toyoda H, Kumada T, Tada T. Changes in patient backgrounds may increase the incidence of HCC after SVR in the era of IFN-free therapy for HCV. Hepatology 2016;64:1818-9. [Crossref] [PubMed]
- Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727-33. [Crossref] [PubMed]
- Kozbial K, Moser S, Schwarzer R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol 2016;65:856-8. [Crossref] [PubMed]
- Cheung MCM, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;65:741-7. [Crossref] [PubMed]
- stanislas.pol@aphp.fr AcsgohcEa. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65:734-40. [Crossref] [PubMed]
- Ioannou GN, Green PK, Beste LA, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 2018;69:1088-98. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]


