
Robotic distal pancreatectomy: can results overcome cost-effectiveness prejudices?
Pancreas surgery was historically considered the most challenging type of surgery for surgeons all over the world. It is related to higher morbidity and mortality rates as compared to other surgical procedures and survival rates for pancreatic adenocarcinoma are still disappointing (1). Few technical innovations have been produced so far and minimally invasive procedures represent the most important addition to the set of surgical strategies to treat pancreatic diseases. The short-term clinical advantages of minimally invasive distal pancreatectomy (DP) in terms of less intraoperative blood loss and shorter postoperative hospital stay were recently confirmed by the DIPLOMA study, a European retrospective propensity score-matched cohort study on minimally invasive versus open DP (MIDP vs. ODP) for pancreatic duct adenocarcinoma (2). Even if the overall survival was reported to be comparable after both procedures, this study highlighted that the oncological safety of MIDP for pancreatic duct adenocarcinoma remains unclear, as despite higher R0 resection rates, Gerota’s fascia was resected less often and lymph node retrieval was lower in MIDP. Robotic pancreatic surgery is the most recent frontier of minimally invasive surgery applied to the surgical treatment of pancreatic tumors and so far has been considered a pioneering approach due to both its novelty and the few available data, even though the use of robotic technology to perform a robotic DP (RDP) was first reported by Melvin in 2003 (3). A recent meta-analysis comparing laparoscopic and robotic DP showed that RDP is associated with a higher rate of spleen preservation, reduced hospital stay and a decreased conversion rate, without any increase of the rate of post-operative complications (4). Although the decision to perform a splenectomy is primarily dictated by the oncological indication, it is well known that spleen preservation is related to less post-operative complications and particularly the overwhelming post-splenectomy infection syndrome. Post-operative pancreatic fistula (POPF) remains an unsolved problem and the most dangerous post-operative complication after DP. It is a topic of great interest, with an incidence reported to be as high as 47% in some series, independently from the technique used to close the pancreatic stump (5). The incidence of POPF is a multifactorial event, related to both technical, vascular and mechanical factors, such as parenchymal stiffness (6). A meta-analysis comparing the outcomes of 2,286 patients from sixteen studies who received either stapler closure (671 patients) or suture closure (1,615 patients) after DP showed a large variation in PF incidence, ranging from 0% to 40.0% for stapler closure and 9.3% to 45.7% for suture closure (7). The study demonstrated that no significant differences exist between suture and stapler closure of the pancreatic remnant regarding PF or intra-abdominal abscess after DP incidence, however, there is a suggested superiority of stapler closure over suture closure (7). Moraldi et al. in 2018 described their technique for robotic DP and selective Wirsung duct ligation to reduce fistula incidence (8). They reported the outcomes of 21 patients, with a 14% incidence of grade B fistula and a 10% incidence of biochemical leak. This approach is also our choice during robotic DP, and is particularly feasible thanks to the integrated ultrasound (US) image fusion on the da Vinci robotic platform (Intuitive Surgical, Sunnyvale, CA, USA), that allows a precise identification of the main pancreatic duct (Figure 1). The combination of Harmonic ACE (Ethicon, Somerville, NJ, USA) to seal small pancreatic ducts and selective ligation of the main duct seems to reduce the incidence of grade B POPF. This kind of dissection can be approached with a sort of “pancreatic hanging maneuver” with a clear view of the transection plane and safe control of the vascular structures (Figure 2).
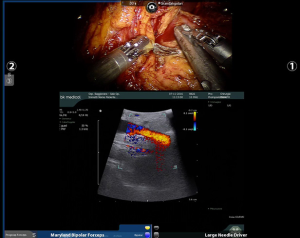
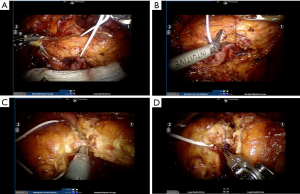
In their interesting multi-institutional analysis, Rodriguez and colleagues (9) raised a crucial question: which surgical approach provides a cost-effective management of patients that require a DP? The study included 89 patients who underwent DP in a three-year interval at two French tertiary referral institutions, highly specialized in minimally invasive and pancreatic surgery. In detail, 21 were robotic (RDP), 25 laparoscopic (LDP), and 43 open (ODP) procedures. As expected, the cost of operating room occupation and instrumentation were lower in the ODP group, but the post-operative hospital stay and parenteral feeding were more expensive after traditional open DP. Moreover, both surgical complications (≥ Clavien-Dindo IIIA) and non-surgical morbidities occurred less frequently in the robotic group in a statistically significant fashion. In terms of surgical efficacy, reduced blood loss (P=0.002) and comparable rates of R0 resections were achieved with the robotic approach.
Taken all together, those results should encourage hospital administrators to invest in minimally invasive strategies to implement the opportunities for patients to obtain a safer resection. It has been demonstrated that outcomes of pancreatic resections can be improved by the experience of the surgeon together with the high volume of patients referred to a center (10). Thanks to its dexterity and to the possibility of implementation of the platform (11), the robot will allow both easier training of young surgeons and re-training of senior surgeons, as compared to laparoscopic surgery, and expand the adoption of minimally invasive surgery in the field of pancreatic surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Klompmaker S, et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Ann Surg 2019;269:10-17. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
- Miyasaka Y, Mori Y, Nakata K, et al. Attempts to prevent postoperative pancreatic fistula after distal pancreatectomy. Surg Today 2017;47:416-24. [Crossref] [PubMed]
- Marchegiani G, Ballarin R, Malleo G, et al. Quantitative Assessment of Pancreatic Texture Using a Durometer: A New Tool to Predict the Risk of Developing a Postoperative Fistula. World J Surg 2017;41:2876-83. [Crossref] [PubMed]
- Zhou W, Lv R, Wang X, et al. Stapler vs suture closure of pancreatic remnant after distal pancreatectomy: a meta-analysis. Am J Surg 2010;200:529-36. [Crossref] [PubMed]
- Moraldi L, Pesi B, Bencini L, et al. Robotic distal pancreatectomy with selective closure of pancreatic duct: surgical outcomes. Updates Surg 2019;71:145-50. [Crossref] [PubMed]
- Rodriguez M, Memeo R, Leon P, et al. Which method of distal pancreatectomy is cost-effective among open, laparoscopic, or robotic surgery? Hepatobiliary Surg Nutr 2018;7:345-52. [Crossref] [PubMed]
- Capretti G, Balzano G, Gianotti L, et al. Management and Outcomes of Pancreatic Resections Performed in High-Volume Referral and Low-Volume Community Hospitals Lead by Surgeons Who Shared the Same Mentor: The Importance of Training. Dig Surg 2018;35:42-8. [Crossref] [PubMed]
- Di Benedetto F, Magistri P, Ballarin R, et al. Ultrasound-Guided Robotic Enucleation of Pancreatic Neuroendocrine Tumors. Surg Innov 2019;26:37-45. [Crossref] [PubMed]


