
Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third leading cause of cancer-related mortality (1). Resulting from virus-related hepatitis, it is regarded as a major public health problem in the Asia-Pacific region.
For these patients of Barcelona Clinic Liver Cancer (BCLC) Stage B, transarterial chemoembolization (TACE) is the most widely used primary treatment and is recommended as first-line therapy in guidelines from the European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Disease (AASLD) (1,2). As a palliative treatment, TACE can achieve a response rate of 55% and a median survival of 20 months (1).
Recently, several studies showed that a surgical resection (SR) provides a better long-term survival rate than TACE in selected patients with multifocal HCC (3-7). However, excessive liver resection is often required for multifocal HCC, and post-hepatectomy liver failure (PHLF) due to the insufficient future liver remnant (FLR) is common. Therefore, SR alone is usually not suitable for patients with multifocal HCC beyond the Milan criteria with insufficient FLR. The strategy that combines SR with radiofrequency ablation (RFA) may act as a substitute for SR for these patients. RFA has gained popularity in the treatment of HCC for its minimal invasiveness and safety (8,9). In the procedure of RFA plus SR (SR-RFA), the superficial tumors or multifocal tumors confined to one lobe are resected, and the nodules near major vascular or deep inside the liver parenchyma are ablated to preserve the FLR as much as possible. Thus far, SR-RFA has been applied primarily for unresectable colorectal liver metastases and has proved to be safe and effective (10-12). It provides curative options for multifocal HCC patients who are traditionally considered inappropriate candidates for SR. However, the effectiveness and safety of SR-RFA for multifocal HCC remains largely unknown. Comparative studies of the therapeutic outcomes between SR-RFA and TACE are still scarce. In this study, we utilize propensity score matching and subgroup analyses to compare the efficacy and safety of SR-RFA with TACE for the treatment of the multifocal HCC.
Methods
Patients
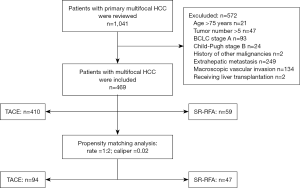
This study was approved by the Institutional Ethics Committee of the Zhongshan Hospital, Fudan University. Retrospective analyses were carried out based on the medical records of patients who were diagnosed with multifocal HCC from January 2009 to December 2015. Only patients who met the following inclusion criteria were enrolled: (I) >18 years and ≤75 years of age; (II) HBV-related HCC (HBV surface antigen-positive, with detectable HBV DNA, or with both the HBV e antibody and HBcAb-positive) but negative for anti-hepatitis C virus (HCV) antibody; (III) multifocal HCC beyond BCLC stage A diagnosed by cytologic/histologic evidence or by noninvasive diagnostic measurements recommended by the EASL (13); (IV) fewer than or equal to 5 nodules; and (V) preserved liver function was classified as Child-Pugh class A without any ascites but patients were intolerant to complete resection. Written informed consents were obtained from all the patients. Patients were excluded if they met any one of the following criteria: (I) a previous history of anti-cancer treatment of HCC; (II) a history of other malignancies; (III) liver functional status of Child-Pugh B/C; (IV) cardiac, pulmonary, cerebral, or renal dysfunction; (V) with extrahepatic metastasis or macroscopic vascular invasion; and (VI) conversion to liver transplantation during the study period.
Definition
The primary endpoint of this study was the overall survival (OS) rate; the secondary endpoint was the safety of SR-RFA. OS was defined as the time from the date of surgery to the date of the last follow-up or the date of death, regardless of the cause of death. Postoperative complications (POC) were defined according to the Clavien-Dindo criteria in the SR-RFA group, and grades IIIb, IV, and V were considered as severe (14). The toxicity of adjuvant TACE was evaluated by the Common Terminology Criteria for Adverse Events version 4.0 in the TACE group.
Treatment
TACE
TACE was performed using the Seldinger technique via percutaneous femoral arterial catheterization as previously reported, and repeated on demand according to clinician judgement (15). The dose of a chemotherapeutic agent, lipiodol, and embolic material deployment were determined according to tumor burden, vascularity and liver function reverse. Routine enhanced computed tomography (CT) scans of the liver were performed to determine the effects 4–6 weeks after treatment. The interval between the two sessions of TACE was 4 weeks. If the CT scan indicated that the tumor had not been fully embolized after four sessions of TACE, the TACE treatment would not be continued. To objectively judge the efficacy of treatment, the modified response evaluation criteria in solid tumors (mRECIST) were applied to determine the response of the tumor to treatment.
Surgical technique
The SR-RFA was performed as follow: A subcostal incision was used for laparotomy, followed by an exploration of the abdomen and pelvis to confirm the absence of an extrahepatic lesion. Intraoperative ultrasound was conducted to identify the tumor location and the number of tumors in addition to the relationship with the vasculature of the liver. As complete resection of all lesions was not possible, combined SR and RFA were performed during one procedure to achieve curative resection. The reasons for the adoption of combination therapy were the bilobar disease (n=17), proximity to major vessels or the bile duct (n=17) and small tumors deep in the liver requiring large resection (n=25). The techniques of SR and RFA in our center have been previously and separately described in detail (16,17). An RFA electrode was inserted into the tumor nodule under ultrasound guidance using the Cool-Tip system (Radionics, Burlington, MA, USA). A single needle or needle cluster was used according to the size of the target tumor (a cluster needle was preferred for tumors larger than 3 cm but smaller than 5 cm). The endpoint of ablation was the complete ablation of both the visible tumors and margins of at least 0.5–1.0 cm in liver parenchyma surrounding the tumors (internally cooled for 12 to 18 minutes).
Postoperatively, any patients (including patients in the SR-RFA group and the TACE group) who met the antiviral therapy criteria of the Asian Pacific Association for the Study of the Liver (APASL) received either lamivudine (100 mg) or entecavir (0.5–1.0 mg) daily. Adefovir (10 mg) was added to those patients who had resistance to either lamivudine or entecavir (18).
Follow-up
Patients were followed up with in our clinic once every month in the first postoperative year and once every 3–4 months thereafter. Liver function tests, serum alpha-fetoprotein (AFP), and hematological parameters were examined. Liver ultrasonography was performed by clinicians who were not involved in and had no access to the treatment information of the patients in this study. A computed tomography scan of the chest, abdomen and pelvis was performed once every 6 months. If tumor recurrence in the liver was suspected, contrast-enhanced CT /Magnetic Resonance Imaging scan or biopsy of the lesions would be performed if clinically indicated. A bone scan was performed to exclude metastasis as necessary.
Treatment of recurrence after SR-RFA
Patients with recurrences after SR-RFA were treated depending on the tumor size, tumor location, number of tumors, and liver function. In short, for localized intrahepatic tumors, liver reresection, RFA, or percutaneous ethanol injection (PEI) were offered. For multiple or diffused intrahepatic recurrences, TACE was offered as a first-line treatment. External beam radiotherapy was applied to lymph nodes or bone metastases.
Propensity score matching analysis (PSM)
PSM analysis was employed to reduce bias in patient selection to investigate the differences between the SR-RFA and TACE groups. Variables showing significant differences or associations with patient selection, including maximal tumor size, the number of tumors, international normalized rate (INR), albumin, and AFP, were comprehensively included in the calculation of the propensity score. The caliper value was set as 0.02. Binary logistic regression with the selected variables was used to generate a propensity score from 0 to 1. The matching algorithm was chosen using nearest-neighbor matching without replacement at a ratio of 1:2.
Statistics
Patients’ baseline characteristics were reported as median (range) or percentage. The Mann-Whitney U test and t-test were employed to compare continuous variables, and the χ2 and Fisher exact test were employed to compare categorical variables. The results of this study were based on the date of the last available follow-up (July 1st, 2017). OS was examined by the Kaplan-Meier method using the log-rank tests. Prognostic factors potentially associated with survival included age, number of tumors, maximal tumor size, serum bilirubin, INR, albumin, alanine aminotransferase, gamma-glutamyl transpeptidase (γ-GT) and AFP. Factors with a P value less than 0.10 in univariate analyses were introduced into the multivariate Cox proportional hazards model to determine an independent impact on OS. Hazard ratios (HR) and 95% CIs were estimated by use of a nonparametric log-rank test with the Cox proportional hazards model. All statistical analyses were conducted using SPSS for Windows Version 24.0 (IBM, NY). A two-tailed P value less than 0.05 was considered statistically significant.
Results
Characteristics of all patients
A total of 469 multifocal HCC patients (TACE: n=410, SR-RFA: n=59) were enrolled. The workflow of patient selection for this study was detailed in Figure 1. The median follow-up period was 27.0 months (range, 18.0–87.2 months) in the SR-RFA group and 24.3 months (range, 18.0–84.4 months) in the TACE group. In the SR-RFA group, 59 patients had 74 tumor nodules resected (range, 2.5–15.0 cm with a median size of 5.0 cm) and 90 tumor nodules ablated (range, 0.5–4.5 cm with a median size of 1.5 cm). Twenty-one patients had 2 or 3 tumors ablated while other patients (n=38) had one tumor nodule ablated. In the TACE group, 410 patients had 1,273 tumor nodules in total (range, 1.5–19.2 cm with a median size of 5.3 cm). All patients in the TACE group received TACE 3.2 times on average (range, 1–9 times with a median of 3 times).
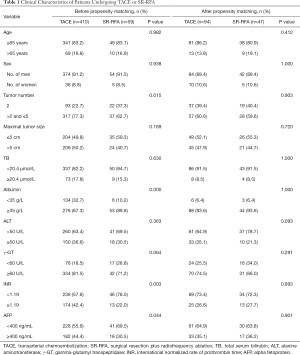
The baseline demographic and clinicopathological data for the 469 patients are displayed in Table 1. The median age of patients was 55 years in the TACE group (range, 20–75 years) and 56 years in the SR-RFA group (range, 28–74 years). There were no significant differences in sex, age, total serum bilirubin or maximal tumor size between the two groups; however, the TACE group was associated with more tumors, prolonged INR, lower serum albumin levels and higher AFP levels (all P<0.05).
Full table
Complications and toxicity
There were 18 (30.5%) POC in the SR-RFA group. Two patients presented with grade IIIa complications, but no severe complications occurred. The most common complications in the TACE group were nausea/vomiting (48.8%, 200/410) and transient hepatic toxicity as characterized by elevations in alanine aminotransferase levels up to 41.2% (169/410), elevations in total bilirubin up to 31.5% (129/410), and elevations of γ-GT up to 18.5% (76/410). No grade 3 or 4 adverse events occurred according to the Common Terminology Criteria for Adverse Events version 4.0. The 30-day mortality rate in the TACE and RFA-SR groups were 1.22% (5/410) and nil, respectively (P=0.861).
Survival analysis of the entire study population
Nineteen patients (32.2%) in the SR-RFA group and 256 patients (62.4%) in the TACE group died during the following-up period. Patients who received SR-RFA had a significantly longer OS compared to patients treated with TACE (P<0.001) (Figure 2A). The 1-, 2- and 3-year OS rates for SR-RFA were 81.5%, 68.3% and 64.3%, whereas the corresponding OS rates for TACE were 59.3%, 35.5% and 24.4%, respectively. The median survival time was 16 months in the TACE group. The median survival has not yet been reached in the SR-RFA group.

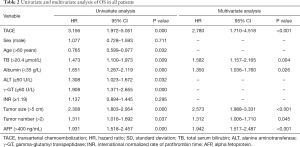
Potential prognostic factors that were associated with OS included treatment strategy, preoperative total bilirubin (TB), albumin, alanine aminotransferase, γ-GT, maximal tumor size, AFP level and Child-Pugh scores. In the multivariate Cox modelling, 5 independent prognostic factors for the survival of multifocal HCC patients were identified. TACE (HR, 2.780; 95% CI, 1.710–4.518; P<0.001), maximal tumor size larger than 5 cm (HR, 2.573; 95% CI, 1.988–3.331; P<0.001), preoperative AFP level higher than 400 ng/mL (HR, 1.942; 95% CI, 1.517–2.487; P<0.001), preoperative albumin lower than 35 g/L (HR, 1.350; 95% CI, 1.036–1.760; P=0.026), and preoperative TB higher than 20.4 µmol/L (HR, 1.582; 95% CI, 1.157–2.165; P=0.004) were associated with poor survival (Table 2).
Full table
Characteristics and survival analysis of patients in the propensity model
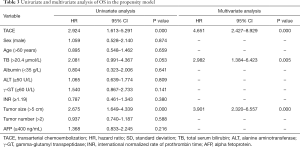
The characteristics of patients selected by the propensity model are presented in Table 1. Each variate associated with survival was well-matched (all P>0.05). In the propensity model of TACE and SR-RFA; the OS rate of the SR-RFA group was significantly longer than the TACE group (P<0.001) (Figure 2B). The 1-, 2- and 3-year OS rates were 81.8%, 68.7% and 63.4%, whereas those in the SR-RFA group were 59.3%, 36.1% and 19.4% in the TACE group, respectively. The multivariate Cox proportional hazards model identified TACE as an independent predictor of poor OS (Table 3).
Full table
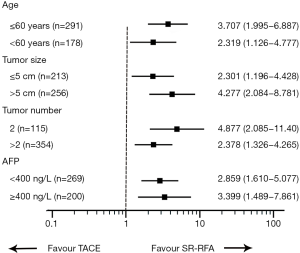
Subgroup analysis of the entire study population
A subgroup analysis was conducted to clarify the survival benefits of SR-RFA compared with TACE in the entire population (Figure 3). In all subgroups, the SR-RFA group achieved a significant improvement in survival compared to the TACE group. SR-RFA provided a clinical benefit in all exploratory subgroup analyses, despite some patients presenting with characteristics associated with poor prognosis, including maximal tumor size >5 cm, tumor number >2, and high levels of AFP.
Discussion
Although TACE is recommended by the AASLD guidelines as a first-line treatment for patients with multifocal HCC of BCLC stage B, recent studies have reported that SR offers better long-term survival than TACE in selected patients with intermediate and advanced-stage HCC (4,5,19-21). SR is a viable treatment option for BCLC stage B HCC if the tumor location and liver function allow for resection (22,23). Despite radical treatment providing better survival time than TACE, many patients with multifocal HCC are not suitable for SR because of the high risk of hepatic failure after resection. Resection of multiple tumors can also cause resection of a large amount of liver tissue. Posthepatectomy liver failure due to future liver remnant insufficiency is a common and feared complication after extensive liver resection. Underlying chronic liver disease (liver fibrosis or cirrhosis) often results in insufficient future liver remnant after extensive liver resection, and PHLF is prone to occur in this context. In recent years, SR-RFA has emerged as an alternative treatment for these patients and a series of small-scale studies (12,24-28) show that SR-RFA offers a promising future in the treatment of multifocal HCC. It provides the opportunity for curative treatment in certain patient populations. To our knowledge, this study involved the largest number of patients regarding SR-RFA for multifocal HCC.
The survival of multifocal HCC patients undergoing TACE is usually very limited. The median OS was only approximately 16.2–22.6 months (1). SR-RFA can significantly improve survival. Our study showed that SR-RFA provided a significantly better OS than TACE in the entire study population (P<0.001). Since prognostic factors associated with OS were unbalanced between the two groups, PSM was applied to reduce bias. For instance, in the entire study population, patients in the TACE group had worse pathogenic conditions (poorer liver function and more tumors). After PSM, potential significant prognostic factors were well-balanced, and the results were more credible. Similar results were found (P<0.001), and patients who received SR-RFA had a 44.0% increase in the 3-year OS rate compared to those in the TACE group in the propensity model. These results were consistent with the findings reported in other retrospective studies with smaller sample sizes (24,25,28,29). This study utilizes a propensity-matching analysis with an adequate statistical power to show the independent impact of SR-RFA by controlling commonly known prognostic factors.
Subgroup analyses were done according to age, tumor number, maximal tumor size, and AFP levels in all patients with multifocal HCC. The hazard ratio of these analyses was more than 1, suggesting that SR-RFA provided a benefit to each subpopulation, including those patients who typically fare worse. As much as the tumor location and liver function reserve will allow, patients older than 60 with more than two tumors larger than 5 cm may also achieve a longer survival through SR-RFA compared with TACE.
This study has several limitations. First, the retrospective nature is prone to potential bias. PSM cannot eliminate the selection bias. Second, all the patients in this study had HBV-related HCC. Whether the results of this study can be extrapolated to patients with other etiological factors is unknown. Third, this study is underrepresented as it is a single-center study carried out in China. To further verify the conclusions made here, other well-designed, multicenter, randomized controlled trials are needed.
In conclusion, SR-RFA provided better long-term survival than TACE for patients with unresectable, multifocal HCC beyond the Milan criteria. SR-RFA may serve as an alternative treatment for patients with multifocal HCC in a selected patient population.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (No. 81372650; No. 81572296) and Zhongshan Science & Technology Innovation Fund (2015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Ethics Committee of the Zhongshan Hospital, Fudan University. Written informed consents were obtained from all the patients.
References
- EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Liu PH, Hsu C, Hsia C, et al. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 cm in a Propensity Score Model. Ann Surg 2016;263:538-45. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong W, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Galle PR, Tovoli F, Foerster F, et al. The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol 2017;67:173-83. [Crossref] [PubMed]
- Yin L, Li H, Li A, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol 2014;61:82-8. [Crossref] [PubMed]
- Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015;62:440-51. [Crossref] [PubMed]
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-9. [Crossref] [PubMed]
- Xu XL, Liu X, Liang M, et al. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology 2018;287:461. [Crossref] [PubMed]
- Sasaki K, Margonis G, Andreatos N, et al. Combined resection and RFA in colorectal liver metastases: stratification of long-term outcomes. J Surg Res 2016;206:182-9. [Crossref] [PubMed]
- Mima K, Beppu T, Chikamoto A, et al. Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol 2013;18:847-55. [Crossref] [PubMed]
- Qiu J, Chen S, Wu H. Long-term outcomes after hepatic resection combined with radiofrequency ablation for initially unresectable multiple and bilobar liver malignancies. J Surg Res 2014;188:14-20. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet J, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Jiang J, Hu H, Liu R, et al. Nomogram for individualized prediction of recurrence after postoperative adjuvant TACE for hepatitis B virus-related hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e7390. [Crossref] [PubMed]
- Chen R, Gan Y, Ge N, et al. Transarterial Chemoembolization versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Resection within Barcelona Clinic Liver Cancer Stage 0/A: A Retrospective Comparative Study. J Vasc Interv Radiol 2016;27:1829-36. [Crossref] [PubMed]
- Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer 2001;91:1479-86. [Crossref] [PubMed]
- Sarin SK, Kumar M, Lau G, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. [Crossref] [PubMed]
- Makuuchi M, Sano K. The surgical approach to HCC: Our progress and results in Japan. Liver Transplantation 2004;10:S46. [Crossref] [PubMed]
- Kim H, Ahn S, Hong S, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg 2017;104:1045-52. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo A, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Ho MC, Hasegawa K, Chen X, et al. Surgery for Intermediate and Advanced Hepatocellular Carcinoma: A Consensus Report from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer 2016;5:245-56. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Itoh S, Morita K, Ueda S, et al. Long-Term Results of Hepatic Resection Combined with Intraoperative Local Ablation Therapy for Patients with Multinodular Hepatocellular Carcinomas. Ann Surg Oncol 2009;16:3299-307. [Crossref] [PubMed]
- Choi D, Lim H, Joh J, et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol 2007;14:3510-8. [Crossref] [PubMed]
- Eisele RM, Zhukowa J, Chopra S, et al. Results of liver resection in combination with radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol 2010;36:269-74. [Crossref] [PubMed]
- Zhang T, Zeng Y, Huang J, et al. Combined resection with radiofrequency ablation for bilobar hepatocellular carcinoma: a single-center experience. J Surg Res 2014;191:370-8. [Crossref] [PubMed]
- Cheung TT, Ng K, Chok K, et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: prognosis and outcomes. World J Gastroenterol 2010;16:3056-62. [Crossref] [PubMed]
- Espinosa W, Liu Y, Wang C, et al. Combined resection and radiofrequency ablation versus transarterial embolization for intermediate-stage hepatocellular carcinoma: A propensity score matching study. J Formos Med Assoc 2018;117:197-203. [Crossref] [PubMed]


