
Edible hybrid microbial-electronic sensors for bleeding detection and beyond
Introduction to edible electronics
Edible electronic devices are orally deployable electrified medical devices that have great potential to diagnose and treat many types of diseases. Interest in edible electronic devices has accelerated in recent years for several reasons. First, the role of gut health on overall health is becoming apparent. Microbiome composition influences metabolism, immunity, and cognitive function. The gut microbiome is also a valuable biomarker for distinguishing between healthy and diseased states. Distributed and longitudinal surveillance may could detect disorders such as inflammatory bowel disease (IBD), Crohn’s disease, colorectal cancer, or bleeding. Second, the availability of computing platforms and genomic information are becoming more prevalent, cost-effective, and distributed. Miniaturized and low-cost electronics serve as a core technology for designing and fabricating edible electronic devices. Distributed computing platforms can provide a complementary monitoring network to interrogate ingestible medical devices in ambulatory patients. Third, there have been critically enabling advances in materials, non-conventional fabrication, and device integration. Here, Mimee et al. leverage many of these capabilities to create an edible electronic device to detect bleeding in clinically relevant animal models. The key innovation is the use of genetically engineered bacteria that transduce biochemical markers for bleeding into optical signals that can be measured, quantified, recorded, and communicated to external devices outside the body. This unique demonstration of a bio-integrated edible electronic sensor could serve as a modular platform technology that could be leveraged to monitor many other aspects of gut health.
Historical perspective of edible electronic devices
Era of innovations in circuit design
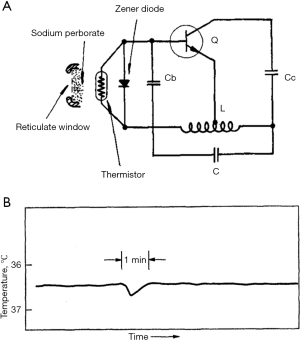
It is important to contextualize the recent device by first gaining a historic perspective of this class of medical devices. Edible electronics were first reported as early as the 1950s. These devices were defined as examples of endoradiosondes, microelectronic devices introduced into the body to record physiological data not otherwise obtainable. Initial advances in edible electronics were predicated on innovations in circuit design and microwave communications, the latter of which enabled data uplink to external devices. Many early devices could measure, transduce, and communicate rudimentary chemical or mechanical physiological information within the gut. Said devices had somewhat obvious potential applications in patient monitoring. Early achievements demonstrated the ability to measure pH, intestinal pressure, and even bleeding (1). In the latter case, the encapsulated device contained an access window doped with sodium perborate, which is a stable source of oxygen (Figure 1). Catalase is an enzyme in the erythrocytes of blood that can catalyze decomposition of hydrogen peroxide. Accordingly, hydrogen peroxide, generated from sodium perborate reservoirs, is decomposed into water and oxygen in an exothermic reaction. Local temperature increases are detected by a nearby thermistor and these data are recorded as a function of time. This early sensing technology provides a roadmap for ingestible sensor design and shares many components that are used in the more recent demonstration by Mimee et al. including sensors, transducers, and packaging.
Era of innovations in systems integration and miniaturization
Advances in systems integration enabled the next phase of innovation in edible electronic devices. For example, the “Ingestible Thermal Monitoring System” was developed by NASA in the 1980s (2). This device could reliably measure core body temperatures of humans in ambulatory environments. On-board Ni-Cd batteries power these devices for up to 72 h while magnetic fields enable remote signal transduction at distances up to 1 m. More recent examples of edible electronics have been enabled by miniaturization of device components. Perhaps the most popular example is an ingestible camera that can be used to image the upper gastrointestinal (GI) tract (3). Other examples include ingestible drug delivery systems and ultra-miniature silicon-based devices for monitoring patient compliance to oral medications (4).
Current work: ingestible sensors that use synthetic biology to detect bleeding
Bleeding in the GI tract is a dangerous and impactful condition that can benefit from early detection. The risks associated with bleeding motivated the design and fabrication of ingestible devices to detect blood (1). While early devices showed promising proof-of-concept, the, the spatiotemporal resolution and dose-dependent signal could be improved. Mimee et al. describe the design and integration of engineered microbes as on-board biosensors to detect both blood and various small molecules (5). In this work, a probiotic strain of E. coli was modified to contain the following genes: a synthetic promoter that is regulated by HrtR, a heme-responsive transcriptional repressor; ChuA, an outer-membrane heme transporter; and luxCDABE, an operon that encodes all the necessary enzymes and substrates required for photoluminescence (6). These components are prepared into a genetic circuit by which extracellular heme is transported into the cell to activate the photoluminescent output in a dose-dependent manner with adequate signal-to-noise ratios. Engineered E. coli sensors are then packaged into reservoirs that can interact with fluid in the gut via a semi-permeable membrane while simultaneously interfacing with optical components (Figure 2). Optical signatures from engineered bacterial sensors are detected using phototransistors interfaced with an on-board low-power luminometer. The optical signal is quantified, digitized, and then wirelessly transmitted using electronics and an antenna on a device that is powered by a 5 mAh battery. The integrated system was reported to be stable in gastric fluid for up to 36 h while the low-power electronics permit operational device lifetimes of up to 6 weeks. Devices loaded with heme-detecting E. coli can detect artificial bleeding in vivo using porcine subjects during a 2 h experiment. This study is compelling because of the intrinsic modularity of the genetically engineered bacterial sensors. Variants of E. coli sensors were genetically engineered to detect other small molecules including acyl-homoserine lactone or thiosulfate.
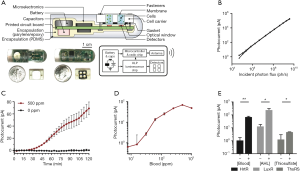
Decoupling the sensing element from the electronics is convenient from both a technology and regulatory perspective. Benign endogenous bacteria with engineered genetic circuits could, in principle, be designed and optimized to detect virtually any small molecule or protein including specific biomarkers for measuring metabolism, detecting GI pathologies, or profiling microbiome composition. Furthermore, it may be possible to multiplex various bacterial sensors on to one ingestible device to measure panels of molecules simultaneously. These data may provide insight into gut health from various perspectives. Decoupling the sensing element from the electronic components can expedite the regulatory approval of these devices as diagnostics for many disease states. Once the initial device is approved in humans, this technology could serve as a predicate device for subsequent technologies using the 510(k) regulatory pathway.
The future of edible electronic sensors
Mimee et al. bring to bear advances in synthetic biology for applications in edible electronic devices. As such, the era of innovations in genetic engineering has now interfaced with endoradiosonde. In the near future, it may be possible to engineer bacterial sensors to measure the concentrate of small molecule metabolites or other markers for metabolic disease or gut health. It may be possible to detect biomacromolecules that serve as anticipatory biomarkers for GI disorders such as Crohn’s disease or IBD. Early diagnosis may improve the ability to intervene, treat, and manage these diseases. Microbial-electronic sensors could also be potentially integrated with on-board therapeutic interventions such as drug delivery reservoirs. The present device uses custom electronic circuits designed and fabricated using off-the-shelf components such as batteries, printed circuit boards, and encapsulation materials. The materials selection process for device components is often motivated by convenience. However, the lack of application-specific materials can subject prospective patients to significant risk associated with device retention or acute toxicity. Next-generation edible electronics may benefit from novel components such as biocompatible batteries, flexible electronics, and biodegradable encapsulation materials (7-9). Edible electronic devices that use flexible, degradable, and biocompatible materials may reduce risk, expedite regulatory approval, and accelerate adoption. Combining advances in synthetic biology with those in biocompatible, biodegradable, and flexible electronics could create a new era in the design of edible electronic devices to diagnose and treat many diseases in the gut and beyond.
Acknowledgements
Funding: The author acknowledges the financial support provided by the following organizations: the National Institutes of Health (R21NS095250), the Defense Advanced Research Projects Agency (D14AP00040), the National Science Foundation (DMR1542196), and the Carnegie Mellon University School of Engineering.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kimoto S, Watanuki T, Hori M, et al. Endoradiosonde for bleeding detection. Med Electron Biol Eng 1964;2:85-7. [Crossref] [PubMed]
- Cutchis PN, Hogrefe AF, Lesho JC. The ingestible thermal monitoring system. Johns Hopkins APL Tech Dig 1988;9:16-21.
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [Crossref] [PubMed]
- Hafezi H, Robertson TL, Moon GD, et al. An ingestible sensor for measuring medication adherence. IEEE Trans Biomed Eng 2015;62:99-109. [Crossref] [PubMed]
- Mimee M, Nadeau P, Hayward A, et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018;360:915-8. [Crossref] [PubMed]
- Craney A, Hohenauer T, Xu Y, et al. A synthetic luxCDABE gene cluster optimized for expression in high-GC bacteria. Nucleic Acids Res 2007;35:e46. [Crossref] [PubMed]
- Kim YJ, Wu W, Chun SE, et al. Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices. Proc Natl Acad Sci U S A 2013;110:20912-7. [Crossref] [PubMed]
- Bettinger CJ. Materials Advances for Next-Generation Ingestible Electronic Medical Devices. Trends Biotechnol 2015;33:575-85. [Crossref] [PubMed]
- Bettinger CJ. Advances in Materials and Structures for Ingestible Electromechanical Medical Devices. Angew Chem Int Ed Engl 2018;57:16946-58. [Crossref] [PubMed]


