
NGM282: a step forward in the nonalcoholic steatohepatitis treatment landscape?
Nonalcoholic fatty liver disease (NAFLD) is becoming the leading cause of liver disease worldwide, and the prevalence is still increasing, especially during the developmental age (1-3). NAFLD is defined in the presence of increased hepatic fat content not explained by at risk alcohol intake, and is epidemiologically associated with obesity, insulin resistance and metabolic disorders. It spans a histological spectrum ranging from simple uncomplicated steatosis, to nonalcoholic steatohepatitis (NASH), which is characterized by hepatocellular damage and inflammation and may progress to advanced liver disease and hepatocellular carcinoma. The pathogenesis of NASH is multifactorial, but an important role is likely played by inflammation triggered by predisposing genetic factors, oxidative stress, and bacterial products derived from the gut (4). Recent data seem to suggest that hepatic lipid accumulation itself may also be involved in NASH by inducing lipotoxicity and driving secondary inflammation, leading to activation of fibrogenesis (5,6). Although lifestyle modification, weight loss and increased physical activity are effective in improving liver histology in patients with NASH, these goals are difficult to achieve and maintain. Unfortunately, pharmacological treatments for this condition are not yet available, while they are urgently needed to tackle a possible global rise in liver disease related mortality in the coming years (2). The drugs under development either tackle hepatic fat accumulation, or act downstream by modulating inflammation and fibrogenesis (6). Among drugs that influence hepatic lipid metabolism, particularly promising results have been reported for agonists of Farnesoid X receptor (FXR) such as obeticholic acid, which in a phase 2 trial in patients with NASH was associated with a higher rate of amelioration of hepatic steatosis and fibrosis as compared to placebo (7).
In a recent study, Harrison and coworkers examined the impact of NGM282, a synthetic Fibroblast growth factor 19 (FGF19) analogue on the amelioration of hepatic fat accumulation and liver damage in phase 2 randomized placebo-controlled trial in patients with NASH (8). They randomized 82 patients to either placebo or NGM282 3 mg or 6 mg daily by subcutaneous injections. They found that NGM282 at 3mg and 6mg dose achieved the major outcome that is clinically significant improvement in liver fat, in three quarters of patients. Specifically, exposure to NGM282 resulted in a remarkable average 10% decrease in hepatic fat content, as estimated by magnetic resonance imaging proton fat fraction (MRI-PDFF), corresponding to a 48–60% relative decrease from baseline, whereas the placebo was not effective in ameliorating this outcome. Reduction of hepatic fat was more pronounced in those with at least 20% liver fat at baseline that is in the patients with more severe disease. Remarkably, reduction of hepatic fat correlated with amelioration of aminotransferases, and some markers of fibrogenesis previously associated with fibrosis severity (TIMP1 and pro-C3 collagen fragment). It also correlated with suppression of circulating levels of C4, an intermediate metabolite of the synthesis of bile acids, reflecting the inhibition of one of the biological pathways targeted by the investigational compound.
Several points render this study innovative. The first one is the choice of the main outcome, based on an absolute reduction of hepatic fat content >5%, which was previously associated with amelioration of disease activity in patients who lost weight. This is supported by recent evidence suggesting that hepatic fat accumulation plays a causal role in NAFLD progression, and may represent a therapeutic target (5,6). Supporting this choice, amelioration of hepatic fat correlated with reductions of liver enzymes and some markers of fibrogenesis. Furthermore, preliminary data suggest that NGM282 treatment would also lead to amelioration of liver disease activity and histological fibrosis (9). If the follow-up studies will confirm that achieving this outcome will be associated with improvement of histological damage and most importantly, a reduction in liver-related events, in the future it will be possible to noninvasively monitor the response to drugs targeting this pathogenetic step and liver damage, avoiding the need of repeating liver biopsies.
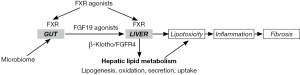
The second novelty relies in mechanism of action, which exploits the activity of a gastrointestinal hormone, FGF19, on hepatic bile acids and lipid metabolism. Furthermore, the peptide has been engineered in order to lose the ability to induce cell proliferation, as amplification of this gene has been linked with hepatocellular carcinoma development (10). This is the first clear demonstration that modulation of the gut-liver axis can lead to amelioration of NASH. Furthermore, FGF19 is induced by FXR in the intestine (11), and treatment with FXR agonists increases FGF19 circulating levels, although their biological activity may be at least partially independent by activation of this pathway. However, NGM282 has the advantage of competing with FGF19 proliferative activity, which would reassure concerning the long-term safety.
Interestingly, levels of FGF19 and of its coreceptor β-Klotho have previously been reported to be reduced in pediatric NASH (12,13). What we still need to know it’s the role of hepatocellular Fibroblast growth factor 4 (FGFR4) and its co-receptor β-Klotho in the pathogenesis of NASH. Specifically, the precise mechanism by which activation of this signaling pathway leads to reduction of hepatocellular fat content and likely of inflammation and fibrosis as a consequence, remains to be clarified. Although we do know that amelioration of liver fat and damage in patients exposed to NGM282 correlates with inhibition of bile acid synthesis, whether reduction in bile acids toxicity explains the current findings is not known. Whether genetic variation in the FGF19-FGFR4-β-Klotho pathway influences the susceptibility to NASH and can mimic the effect of the drug is still unknown. And also, what are the extra-hepatic effects of the drug? Is there a direct action of appetite control, adipose tissue function, or the weight loss observed at 6 mg dose is related to gastrointestinal side effects?
There are some caveats that need to be kept in mind. As observed for FXR agonists, NGM282 exposure determined an increase in circulating LDL cholesterol that may foster atherogenesis and is particularly worrisome in patients at high cardiovascular risk such as those with NASH. Reassuringly, co-administration of statins seems safe, effective in limiting this unwanted effect, and was exploited in further studies (9). Furthermore, the long-term tolerability of gastrointestinal disturbances and the possible development of neutralizing antibodies are of concern.
Despite the aforementioned limitations, all in all this study reports promising results suggesting that modulation of the FGF19-FGFR4-β-Klotho axis may improve hepatic fat accumulation and liver damage in patients with NASH (as depicted in Figure 1), and has the potential to be evaluated as a first enterokine-based approach to treat a liver disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. [Crossref] [PubMed]
- Estes C, Anstee QM, Teresa Arias-Loste M, et al. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896-904. [Crossref] [PubMed]
- Nobili V, Svegliati-Baroni G, Alisi A, et al. A 360-Degree Overview Of Paediatric Nafld: Recent Insights. J Hepatol 2013;58:1218-29. [Crossref] [PubMed]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836-46. [Crossref] [PubMed]
- Dongiovanni P, Stender S, Pietrelli A, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med 2018;283:356-70. [Crossref] [PubMed]
- Pelusi S, Valenti L. Hepatic fat as clinical outcome and therapeutic target for nonalcoholic fatty liver disease. Liver Int 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956-65. [Crossref] [PubMed]
- Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174-85. [Crossref] [PubMed]
- Harrison SA, Rossi S, Bashir M, et al. NGM282 improves fibrosis and NASH-related histology in 12 weeks in patients with biopsy-confirmed NASH, which is preceded by significant decreases in hepatic steatosis, liver transaminases and fibrosis markers at 6 weeks. J Hepatol 2018;68:S75-6. [Crossref]
- Zhou M, Wang X, Phung V, et al. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res 2014;74:3306-16. [Crossref] [PubMed]
- Moschetta A. Nuclear receptors and cholesterol metabolism in the intestine. Atheroscler Suppl 2015;17:9-11. [Crossref] [PubMed]
- Nobili V, Alisi A, Mosca A, et al. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int 2018;38:342-9. [Crossref] [PubMed]
- Alisi A, Ceccarelli S, Panera N, et al. Association between Serum Atypical Fibroblast Growth Factors 21 and 19 and Pediatric Nonalcoholic Fatty Liver Disease. PLoS One 2013;8:e67160. [Crossref] [PubMed]


