Accuracy of preoperative CT liver volumetry in living donor hepatectomy and its clinical implications
Introduction
The waiting list mortality of patients with end-stage liver disease in most deceased donor-based programs remains significant. With technical refinement, standardization and success of live donor liver transplantation (LDLT), the procedure has proved equally efficacious alternative. However, donor safety with adequate remnant and adequate graft recipient weight ratio (GRWR) remain the utmost concerns in LDLT. An accurate preoperative volumetric assessment of the donor liver is important for both these important considerations (1-3).
In addition to general surgical fitness of the donor, donor hepatectomy requires detailed preoperative assessment of liver steatosis, vascular and biliary anatomy and volumetric assessment of the future liver remnant and liver graft. A liver remnant volume of 30–35% of the original volume is required for the donor safety whereas a minimum of 40% of the standard liver mass, or more than 0.8 GRWR is required for the recipient (3,4). With introduction of automated software for liver volume calculation, volumetric assessment has become less time consuming but their validation in clinical practice is still lacking (5). Although manual CT volumetry is the gold standard, it is time consuming (6). So, this study using semiautomatic method was done to know the over or underestimation of liver volume by CT scan with respect to intraoperative actual graft weight (AGW) in different types of living donor hepatectomy.
Methods
Study population
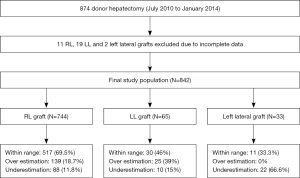
A total of 874 LDLT were performed from July 2010 to January 2014. Preoperative CT volumetric data was not available for 11 right lobe (RL), 19 left lobe (LL) and 2 left lateral sector (LLS) grafts, which were excluded from the study. The study cohort consisted of 842 consecutive donors who underwent hepatectomy which included 744 RL, 65 LL and 33 LLS grafts (Figure 1). All donors were adults between the ages of 18 to 55 years. They were evaluated by the same team of radiologists, and surgeries performed by the same team of surgeons. All donors underwent a detailed preoperative evaluation according to our institutional protocol. The protocol consisted of pre-screening to select 18–55 years old, blood group matched relatives followed by a 4-phase evaluation process. Phase 1 consisted of clinical and laboratory tests for systemic function, viral serology, screening for hepatic steatosis by CT scan ± liver biopsy. Those with liver attenuation index (LAI) <0 were rejected, 0–5 underwent a liver biopsy and >5 were accepted. Upper limit of acceptable macrosteatosis was less than 20%. Phase 2 consisted of a contrast CT scan of the abdomen with a triphasic study of liver for vascular anatomy and volumetry (minimum acceptable GRWR 0.7, remnant 32%) and magnetic resonance cholangiopancreatography for biliary anatomy. Phase 3 consisted of tests for systemic evaluation, and Phase 4 consisted of cardiology, pulmonology, gynaecology (in females), psychiatric clearances and legal committee approval. Liver biopsy was done, where applicable, only when donor volumes were found to be adequate.
CT angiography protocol
All CT angiography abdomen were performed with a multi-detector CT scanner (SOMATOM Definition Flash, 256, Siemens, Germany), before and after intravenous administration of a iodine contrast medium (15 mL/kg) at a concentration of 350 mg I/mL (OMNIPAQUE 350, GE Healthcare, Shanghai, China) through an antecubital vein of the arm, using an automatic double-syringe injector (Stellant, MedRad, Indianola, PA, United States) at a flow rate of 3–4 mL/second, followed by a bolus of 50 mL of saline at the same flow rate. In all cases, dynamic examination was performed, using a bolus tracking technique. The complete scan was done in single breath hold to avoid breathing artifact. The following parameters were used: thickness 3 mm; increment 11 mm; tube voltage 120 kV; collimation 64×0.625; pitch 0.9 for the pre-contrast, venous and delayed phase scan, 0.5 for the arterial phase scan; rotation time 0.75 seconds.
CT volumetry protocol
Transverse images of the portal-venous phase (slice thickness of 5 mm and increment of 5 mm) were used for CT volumetry because hepatic veins were depicted with maximum contrast in this phase (7,8). A semi-automated interactive commercial software called AW Volume share 6 (GE Healthcare; Chicago, Illinois, USA) was used. All major vessels such as the extrahepatic portal vein in the area of the porta hepatis and inferior vena cava, as well as larger fissures, gall bladder and the hepatic ligamentum teres were manually excluded from total liver volume analysis because they have no metabolic function and therefore cannot be added to the volume of the graft .The images were uploaded, and then the outline of the entire liver was determined between liver tissue and surrounding fatty tissue. The liver outline was determined by pencil trace method in cranio-caudal direction. False-positive and false-negative extractions were corrected using manual correction tools.
Thereafter, the volumetric reconstruction of the liver and the quantification of total liver volume were obtained. The radiological plane of transection was drawn along the middle hepatic vein (MHV) to delineate the RL and the LL of the liver (Figure 2). The volume of the caudate lobe was calculated separately. The following volumes are calculated: total liver volume, RL including MHV, RL excluding MHV, LL including MHV, LL excluding MHV. While calculating the GRWR for RL graft, RL excluding MHV is taken as graft weight whereas for LL graft, LL including MHV is takes as graft weight. For left lateral segment graft, the radiological plane of transaction was drawn through the falciform ligament caudally and close to the ostia of left hepatic vein cranially and volume of left lateral segment is calculated.
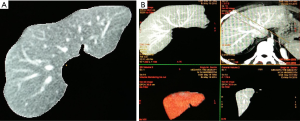
The choice of graft was mainly based on preoperative recipient sickness, graft to recipient body weight ratio (GRWR), remnant volume in donor and vascular anatomy. Three types of RL graft was used based on the length of MHV retrieved (extended RL graft, with partial or subtotal MHV or Modified RL graft with Segment 5 and 8 veins reconstructed on bench) (9). LL graft was always retrieved with MHV. The actual surgical plane of parenchymal transection varied from radiological plane depending on the type of graft retrieved. In left lateral segment graft, the surgical plane of transection was 1 cm right to the falciform ligament.
Measurement of intraoperative graft weight
After retrieval of the liver graft, AGW was measured on the back table after perfusing the graft with Belzer UW solution® (Durant pharma) at 4 degree C, once the returns were clear.
Statistical methods
Descriptive analysis of quantitative data was expressed as means and standard deviation. Categorical data were presented as absolute numbers and percentage. Paired t-test was used to compare the mean of estimated graft weight (EGW) and AGW for each RL, LL and LLS graft. Pearson correlation coefficient was used to study the correlation between EGW and AGW for all three types of liver graft weight.
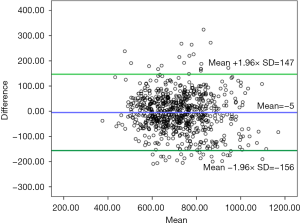
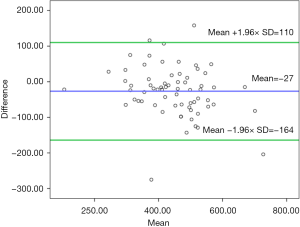
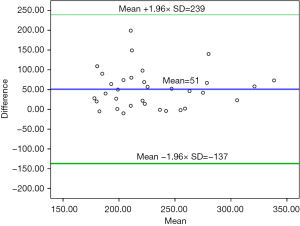
Bland Altman plot was created by plotting the difference between EGW and AGW against mean of EGW and AGW and the 95% confidence interval (mean ±1.96× SD) was used for assessing the level of agreement between two methods.
Percentage deviation was defined as the difference between the AGW and EGW divided by AGW and multiplied by 100 [(AGW−EGW) ×100/AGW] (negative deviation = overestimation; positive deviation = underestimation). Preoperative EGW was converted to graft weight with conversion ratio of 1 (1 mL = 1 gm weight). A two tailed P value of <0.05 was considered significant. All analysis was done using SPSS 20 version (Chicago, IL, USA).
Results
Out of 842 donors, 392 (46.5%) were males and 450 (53.5%) females with a mean age of 34±12 years.
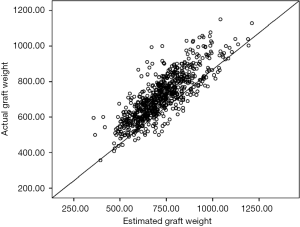
RL graft: there was no statistically significant difference between mean of EGW and AGW in RL (722±134 vs. 717±126 gm; P=0.06). The correlation between EGW and AGW for RL was very strong and statistically significant (r=0.82, P<0.001). The scatter plot between AGW and EGW for RL graft is shown in Figure 3. Out of 744 RL grafts, 517 (69.5%) were within range, 139 (18.7%) were overestimated by preoperative CT and 88 (11.8%) were underestimated by CT volumetry. The Bland Altman graph showed that the 95% limits of agreement range from −156 to +147 (Figure 4).
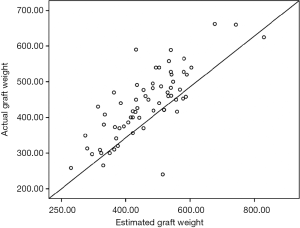
LL graft: although EGW and AGW for LL grafts correlated strongly (r =0.81, P<0.001), mean of EGW was significantly high as compared to mean of AGW (460±118 vs. 433±102 gm; P=0.003). The scatter plot between AGW and EGW for LL graft is shown in Figure 5. Thirty of 65 LL grafts (46%) were within range, 25 (39%) were overestimated and 10 (15%) were under estimated by CT volumetry. The Bland Altman graph showed that the 95% limits of agreement range from −164 to +110 (Figure 6).
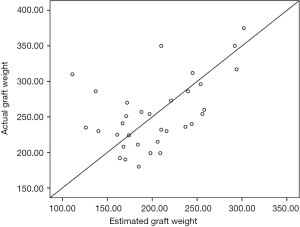
Left lateral segment graft: mean of EGW was significantly low as compared to mean of AGW in LLS graft (203±48 vs. 254±49 gm; P<0.001). The correlation between EGW and AGW for LLS graft was moderate and statistically significant (r =0.49, P=0.003). The scatter plot between AGW and EGW for LLS graft is shown in Figure 7. CT does not overestimate LLS grafts. Twenty-two (66.6%) were underestimated by CT volumetry and 11 (33.3%) of LLS grafts were within range as predicted by CT preoperatively. The Bland Altman graph showed that the 95% limits of agreement range from −137 to +239 (Figure 8).
Discussion
Manual CT volumetry, though labor intensive, is currently the gold standard for volumetric analysis (1,6,10). Automated methods of hepatic volume measurement with CT scans (2,3,11-13) provide acceptable measurement (11,14,15) and reduce dramatically the time required for volumetry assessment. However, these automated volumetric analytical methods tend to overestimate volume as compared to manual methods (2,3,7,11,16). Moreover, all these studies have small number of cases, majority are for RL graft with very few in left and left lateral segments grafts (5,17,18).
Majority of the transplant centers regard 1 cc of liver on pre-operative volumetry to be equal to 1 gram of liver with the assumption that the mean density of healthy liver tissue is 1.00 g/mL (19-21). So preoperatively calculated volumes of both liver lobes have been equated with their respective weights (1,19-21). Hwang et al. measured the amount of blood in human liver grafts and analyzed the correlation between volumetric graft volume and graft weight. They concluded that a conversion factor of 1.22 should be used between the blood-free graft weight and blood-filled graft volume (22,23). However, the utility of a positive fixed conversion factor is doubtful since there may be individual variation, and our results suggest that CT volumetry can both, overestimate and underestimate graft weights. The present study has also taken a conversion factor of 1, between CT estimated graft volumes in cubic cm/milliliters to grams of liver.
There was no significant difference between mean of EGW and AGW for RL graft (P=0.06). However, EGW was significantly high as compared to AGW (overestimation) in LL graft (P=0.003) and EGW was significantly low as compared to AGW (i.e., underestimation) for LLS graft (P<0.001).The Bland-Altman plot showed that level of agreement in LL (−164 to +110) followed by RL (−156 to +147) and LLS grafts (−137 to +239).
There are several factors that account the inaccuracy in EGW. Blood circulating in the intra-hepatic vasculature at the time of imaging studies is associated with graft-volume overestimations when compared to actual blood free grafts measured after retrieval at the back table (7,22,24)). This is due to the fact that the condition of graft on the back table during liver transplant is non- physiological where the liver graft is collapsed after the inner liquids have dissipated through the non-ligated vascular structures (23). Other factors contributing to this discrepancy are: miss-match of actual surgical plane of transection to the radiological plane of liver parenchyma transection plane (1,13,25) and graft dehydration by the University of Wisconsin solution due to its high osmolality (3). Although, EWG is always without MHV in RL graft, the actual RL graft is usually either without MHV (modified RL graft) or with different length of MHV (RL graft with subtotal MHV or partial MHV). This may also account for over or underestimation of RL graft.
In LL graft, the underestimation may be due to non-hepatic tissue like falciform ligament which are excluded while doing CT volumetry. Similarly, the overestimation in LL graft which was more common than underestimation in our series may be due to loss of blood, mismatch between actual surgical plane and radiological plane during volumetry. As the whole scan is done in single breath holding, there is minimal chance of motion artefacts leading to discrepancy in liver volume. In LLS graft, CT always underestimates the volume because actual surgical plane of transection is around 1 cm right to falciform ligament whereas radiological plane is at falciform ligament.
Given the fact that optimal GRWR ≥0.8 being necessary for good outcomes in LDLT, overestimation of graft volume by CT volumetry is a difficult situation, especially in cases with borderline GRWR or high MELD score recipients.
The present study has shown that EGW was significantly high as compared to AGW among LL grafts (EGW =460 vs. AGW =433, P=0.003). Based on our findings, we propose to accept donors with estimated GRWR ≥0.80 for RL grafts and ≥1 for LL grafts. Overestimated EGW and accidental low GRWR can pose recipient risk that can be overcome by keeping adequate safety margins of GRWR, especially in LL grafts. In case GRWR falls to below 0.8 due to a volumetric error, inflow modulation in the form of hemi-portocaval shunt or splenic artery ligation is added.
Another important finding of our study is the underestimation of graft volume by CT volumetry. Although underestimation of RL or LL graft has not much clinical significance in adult patients, but in less than 10 kg recipients, this might be a difficult situation as portal flow in these recipients might be inadequate for a large graft. EGW was significantly low as compared to AGW for LLS graft (EGW =203 vs. AGW =254, P<0.001). Ex vivo or in vivo graft size reduction to ensure a GRWR of <3–3.5 can help overcome this problem. This also solves the issue of over-sized grafts for the abdominal cavities of these small children (usually less than 8 kg), which would otherwise require a mesh for staged closure.
These findings need further validation by fully automated volume measurements in large cohort of patients.
Conclusions
Pre-operative CT volumetry significantly overestimate LL graft weight and underestimate LLS graft weight. This should be factored in when selecting LL graft either by taking higher GRWR or inflow modulation to avoid small for size syndrome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All the donors were evaluated under the set protocol and are approved by legal committee which included members from Government, hospital administration, institutional ethical members (Number: 210/2016) and the above study stands approved.
References
- Kamel IR, Kruskal JB, Warmbrand G, et al. Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol 2001;176:483-7. [Crossref] [PubMed]
- Radtke A, Sotiropoulos GC, Nadalin S, et al. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant 2007;7:672-9. [Crossref] [PubMed]
- Hiroshige S, Shimada M, Harada N, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation 2003;75:1561-4. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997;226:261-9; discussion 269-70. [Crossref] [PubMed]
- Luciani A, Rusko L, Baranes L, et al. Automated liver volumetry in orthotopic liver transplantation using multiphase acquisitions on MDCT. AJR Am J Roentgenol 2012;198:W568-74. [Crossref] [PubMed]
- Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am J Roentgenol 2011;197:W706-12. [Crossref] [PubMed]
- Lemke AJ, Brinkmann MJ, Schott T, et al. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology 2006;240:736-42. [Crossref] [PubMed]
- Schroeder T, Nadalin S, Stattaus J, et al. Potential living liver donors: evaluation with an all-in-one protocol with multi-detector row CT. Radiology 2002;224:586-91. [Crossref] [PubMed]
- Soin AS, Mohanka R, Singla P, et al. Segment IV preserving middle hepatic vein retrieval in right lobe living donor liver transplantation. J Am Coll Surg 2011;213:e5-16. [Crossref] [PubMed]
- Higashiyama H, Yamaguchi T, Mori K, et al. Graft size assessment by preoperative computed tomography in living related partial liver transplantation. Br J Surg 1993;80:489-92. [Crossref] [PubMed]
- Nakayama Y, Li Q, Katsuragawa S, et al. Automated hepatic volumetry for living related liver transplantation at multisection CT. Radiology 2006;240:743-8. [Crossref] [PubMed]
- Karlo C, Reiner CS, Stolzmann P, et al. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol 2010;75:e107-11. [Crossref] [PubMed]
- Radtke A, Sotiropoulos GC, Nadalin S, et al. Preoperative volume prediction in adult live donor liver transplantation: 3-D CT volumetry approach to prevent miscalculations. Eur J Med Res 2008;13:319-26. [PubMed]
- Bégin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP). Surg Endosc 2014;28:3408-12. [Crossref] [PubMed]
- Hermoye L, Laamari-Azjal I, Cao Z, et al. Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology 2005;234:171-8. [Crossref] [PubMed]
- Gondolesi GE, Yoshizumi T, Bodian C, et al. Accurate method for clinical assessment of right lobe liver weight in adult living-related liver transplant. Transplant Proc 2004;36:1429-33. [Crossref] [PubMed]
- Li YC, Hu Y, Zhang MM, et al. Usage of 64-detector-row spiral computed tomography volumetry in preoperative volume prediction in living donor liver transplantation in children. Pediatr Surg Int 2011;27:445-9. [Crossref] [PubMed]
- Wang F, Pan KT, Chu SY, et al. Preoperative estimation of the liver graft weight in adult right lobe living donor liver transplantation using maximal portal vein diameters. Liver Transpl 2011;17:373-80. [Crossref] [PubMed]
- Lemke AJ, Hosten N, Neumann K, et al. CT volumetry of the liver before transplantation. Rofo 1997;166:18-23. [Crossref] [PubMed]
- Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Validation and a comparison of the two techniques. Gastroenterology 1985;88:1812-7. [Crossref] [PubMed]
- Lemke AJ, Brinkmann MJ, Pascher A, et al. Accuracy of the CT-estimated weight of the right hepatic lobe prior to living related liver donation (LRLD) for predicting the intraoperatively measured weight of the graft. Rofo 2003;175:1232-8. [PubMed]
- Salvalaggio PR, Baker TB, Koffron AJ, et al. Liver graft volume estimation in 100 living donors: measure twice, cut once. Transplantation 2005;80:1181-5. [Crossref] [PubMed]
- Hwang S, Lee SG, Kim KH, et al. Correlation of blood-free graft weight and volumetric graft volume by an analysis of blood content in living donor liver grafts. Transplant Proc 2002;34:3293-4. [Crossref] [PubMed]
- Frericks BB, Kiene T, Stamm G, et al. CT-based liver volumetry in a porcine model: impact on clinical volumetry prior to living donated liver transplantation. Rofo 2004;176:252-7. [PubMed]
- Yonemura Y, Taketomi A, Soejima Y, et al. Validity of preoperative volumetric analysis of congestion volume in living donor liver transplantation using three-dimensional computed tomography. Liver Transpl 2005;11:1556-62. [Crossref] [PubMed]