Pretransplant sarcopenia: suffer or fight?
Sarcopenia and liver transplantation (LT)
Sarcopenia is a syndrome characterised by progressive and generalized loss of skeletal muscle mass (SMM) and strength, responsible of physical disability, poor quality of life and death (1). Eastern and Western Sarcopenia working groups agree on the need of both low SMM and low muscle function, for the diagnosis of sarcopenia (2).
LT from Deceased or Living Donor is the only curative treatment for end-stage liver disease (ESLD) with 1- and 5-year survival rates of 84% and 73% (3), respectively. As symptoms of ESLD manifest, there is a progressive decrease in physical activity, and global overall status: secondary sarcopenia (cirrhosis-induced), as high as 30–40% among cirrhotic patients (4,5), is responsible of a reduction of aerobic capacity, severely decreasing 1-year survival after LT down to 53–58% (4,5). Recently Kaido and colleagues (2) published their experience on pretransplant sarcopenia on 72 patients affected by mild cirrhosis (MELD <18) undergoing to living donor liver transplantation (LDLT). All patients at admission underwent to grip strength (GS) measurement and SMM assessment by multi-frequency bioelectrical impedance analysis (BIA): sarcopenia, associating low SMM and low GS, was present in ten patients (14%). Independently from their nutritional status, all patients underwent to preoperative nutritional therapy one week before surgery and feeding jejunostomy realized at the time of LDLT. In this cohort, 1-year patient survival rate was significantly lower for the ten patients affected by sarcopenia than the 62 patients without (60% vs. 94%, respectively, P<0.001). Interestingly, Kaido et al. (2) found that SMM worsened after LDLT and return to preoperative levels only 1 year after LDLT, while GS returned to preoperative levels 6 months later. The conclusion of their research is that pretransplant sarcopenia negatively affects early survival after LT, and long-term rehabilitation program following LT is needed to revert sarcopenia vicious cycle.
There are at least two points on this nice work that needs to be highlighted.
First, the prevalence of sarcopenia observed in this study is 14%, much lower than 30–40% rate reported in western series, and based on the Total Psoas Area as a reflect of SMM. One can argue that the difference relies on the definition of sarcopenia used in this paper (2), associating SMM and GS: but even when considering SMM alone, the prevalence is as low as 18% (13 on 72 patients). Nevertheless, short-term outcomes of patients affected by sarcopenia in this study (2) are similar to western cohort: 60% and 53–58% 1-year survival after LT, respectively (4,5).
The second point, probably the major issue of this study, is the fact that sarcopenia is reversible only after one year following LT. Kaido et al. (2) systematically proposed one week nutritional therapy, before surgery: since LDLT is a scheduled or semi-scheduled intervention, this is logistically feasible. In western countries, liver grafts are mainly issued from deceased donors (DDLT), and the unpredictable character of DDLT implies the absence of any short-term pre-operative planning.
There is still one point, in perspective, which should be raised. The direct relation between sarcopenia and increased mortality rate is sustained by the fact that impairment in cardiovascular fitness [objectively measured through VO2 peak, or 6 minutes walking distance (6MWD)] is strongly associated with higher mortality on waiting list and early after LT (6,7), due to sepsis and rejection (2,4).
Kaido et al. (2) reasonably propose a pre and postoperative nutrition treatment, but no associated physical exercise.
Adapted physical activity (APA) programs are developed to increase VO2 peak and outcomes after major abdominal or cardiac surgery, but no consensus exists on the feasibility of an APA program in patients affected by ESLD, and their potential benefit before and after LT. Moreover, reluctance is justified by the “frail” condition of cirrhotic patients, suffering from sarcopenia, increased fatigability and potentially life-threatening portal hypertension. Within certain limits, ESLD patients could be trained to be more physically fit in order to improve the functional capacity of the cardiovascular system and to enhance preparedness to external stressors as LT. Regular exercise and physical conditioning can cause an increase in 6MWD, VO2 peak and muscle mass, and theoretically lead to a reduced mortality and morbidity after LT.
Evidence from the literature is little: an extensive research on MEDLINE, EMBASE, Google Scholar and the Cochrane Library database through relevant keywords for randomized clinical trials on APA and ESLD allowed us to select two full-text article studies (8,9) and one abstract (10) (Table 1). The intervention was mixed in two studies (9,10) with stationary bicycle and kinesiotherapy or treadmill walking, and combined with a dietary supplementation. One study reported how β-blocker treatment was offered to all patients enrolled (8), while it was selectively offered in two studies (9,10). The length of the intervention was 8 (8), 12 (9) and 14 (10) weeks. Control treatment was dietary supplementation alone in two studies (9,10) and usual care in one study (8). The conclusion of all the three studies is in favour of safety of supervised exercise alone (8) or associated to nutritional therapy (9,10), to increase exercise capacity (8,9), VO2 peak (8,10), leg muscle mass (8,9), Quality of Life (9), reducing fatigue (8) and reducing portal hypertension (10) in cirrhotic patients. Interestingly, none of the three studies (8-10) reported adverse outcome or complications during the exercise period (Table 2).
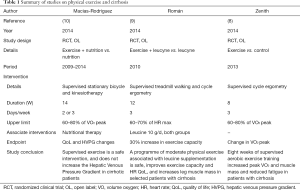
Full table
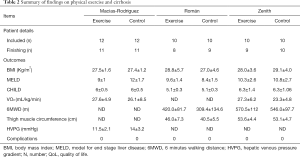
Full table
Even if evidence is derived from small trials, we believe that clinicians should encourage adults with ESLD under β-blocker therapy to participate to regular exercise regimens, combined to nutritional therapy, before and after LT.
Given the potential benefit of these intervention programs among sarcopenic patients—independently of the sarcopenia assessment test—on the early mortality after LT, larger and randomized studies on the association of physical and nutritional interventions before and after LT should be encouraged.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Kaido T, Tamai Y, Hamaguchi Y, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017;33:195-8. [Crossref] [PubMed]
- Available online: http://www.eltr.org/Overall-indication-and-results.html
- Bucur P, Brustia R, Ciacio O, et al. Definition of sarcopenia in cirrhotic patient before liver transplantation. J Hepatol 2013;58:S64. [Crossref]
- Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166-73, 173.e1.
- Prentis JM, Manas DM, Trenell MI, et al. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl 2012;18:152-9. [Crossref] [PubMed]
- Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl 2010;16:1373-8. [Crossref] [PubMed]
- Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920-6.e2. [Crossref] [PubMed]
- Román E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014;59:1966-75. [Crossref] [PubMed]
- Macias-Rodriguez MA, Torre A, Ilarraza-Lo-meli H, et al. Changes on hepatic venous pressure gradient induced by a physical exercise program in cirrhotic patients: a randomized open clinical trial. Hepatology 2014;60:246A.


