Biomarkers are strongly needed in liver transplantation, time to move forward
The European Society for Organ Transplantation (ESOT) has created a consensus platform for evidence-based and best-practice recommendations. At the 2022 ESOT consensus conference, nine topics were chosen for further exploration based on their potential for impact on healthcare, existing research gaps, and incomplete coverage of the topic in current scientific literature (1). Among these topics, molecular biology testing for non-invasive diagnosis of allograft rejection was a focal point, as detailed in the ESOT consensus statement on biomarkers in liver transplantation (LT) (2). We extend our congratulations to the authors of the liver subgroup on their dedicated effort and rigorous approach in translating major unmet clinical needs into four well-defined questions of interest regarding biomarkers for prediction and/or diagnosis of longer-term complications after LT.
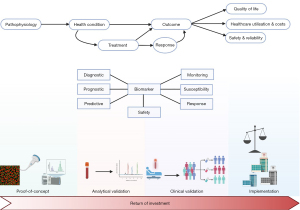
Despite the ongoing advancements in biomarker development and the clear determination of which direction future research should head, there remains a significant gap between these innovations and their routine application in clinical settings, as highlighted in the consensus statement. Biomarker development often faces challenges on the journey ‘from bench to bedside’, with multiple stages and perspectives that are challenging to reconcile (Figure 1). Poste, in a comment published in Nature, pointed out that while “Technologies such as proteomics and DNA microarrays have contributed a voluminous literature of more than 150,000 papers documenting thousands of claimed biomarkers, [...] fewer than 100 have been validated for routine clinical practice” (3). Even when successful, it typically takes an average of seventeen years to implement these developments in clinical practice (4).
The initial step in biomarker development involves laying the groundwork based on the discovery of a potential biomarker closely linked to a disease mechanism. Many candidate biomarkers fail to confirm proposed hypotheses during this proof-of-concept phase and are subsequently not further investigated (5).
Following successful passage of the proof-of-concept phase, the next challenge for candidate biomarkers is analytical validation. This stage involves assessing the reliability and accuracy of a candidate biomarker in patient-derived samples. Early collaboration is crucial to prevent resource and time wastage due to differing approaches that can increase heterogeneity and delay clinical application. The need for early collaboration and streamlining is illustrated by the example of biomarkers for recurrence of hepatocellular carcinoma (HCC) after LT, which we recently studied in a systematic review investigating the use of serum N-glycomics to diagnose HCC recurrence (6). Even when alterations of glycosylation in the same serum protein were considered, diagnostic performance of these alterations could not reliably be compared between studies because of differences in cut-off values, significant study population heterogeneity, and small sample size.
Subsequent clinical validation is necessary to establish correlations between the biomarker result and the clinical condition or outcome. Pilot studies should be replicated in robust and large clinical studies, considering the ultimate objective of the biomarker under study and its potential downstream consequences. This requires a concise definition of the type of biomarker. Diagnostic, prognostic, and predictive biomarkers are related but conceptually different with regard to presence or recurrence of disease, and response to therapy (7). For instance, as mentioned in the consensus statement on recurrence of disease after LT, some genetic factors from recipient, donor or the combination of both, were associated with disease recurrence after LT (2). These genetic factors were investigated as risk factors or susceptibility biomarkers for recurrent disease after LT, which bears large similarities with the concept of a prognostic biomarker (7). However, this does not allow us to use these genetic factors as predictive biomarkers, assessing the likelihood of experiencing recurrent disease after LT. Therefore, clinical validation requires an accurate appraisal of the position of the candidate biomarker within the clinical pathway and its potential to alter this pathway.
Even when the clinical validity of a candidate biomarker is demonstrated, broad implementation in clinical practice can face hurdles due to economic concerns. Balancing the return on investment for companies developing biomarkers and the cost-effectiveness concerns of local governments poses additional barriers to widespread use.
Current advancements provide the necessary tools, resources, and expertise for biomarker development. The field is dynamic, and the time is ripe to consolidate efforts and advance to personalised medicine for LT management. However, achieving this goal requires early and large-scale international collaborative efforts in rigorously designed prospective studies. Only through such endeavours can we effectively address unmet clinical needs after LT with biomarkers that meet the high standards of contemporary medicine.
Acknowledgments
Funding: X.V. was funded by the Foundation against Cancer, Belgium. L.G. was funded by the Research Foundation – Flanders (FWO).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-31/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cillo U, Weissenbacher A, Pengel L, et al. ESOT Consensus Platform for Organ Transplantation: Setting the Stage for a Rigorous, Regularly Updated Development Process. Transpl Int 2022;35:10915. [Crossref] [PubMed]
- Berenguer M, de Martin E, Hessheimer AJ, et al. European Society for Organ Transplantation Consensus Statement on Biomarkers in Liver Transplantation. Transpl Int 2023;36:11358. [Crossref] [PubMed]
- Poste G. Bring on the biomarkers. Nature 2011;469:156-7. [Crossref] [PubMed]
- Monaghan PJ, Robinson S, Rajdl D, et al. Practical guide for identifying unmet clinical needs for biomarkers. EJIFCC 2018;29:129-37. [PubMed]
- Kern SE. Why your new cancer biomarker may never work: recurrent patterns and remarkable diversity in biomarker failures. Cancer Res 2012;72:6097-101. [Crossref] [PubMed]
- Butaye E, Somers N, Grossar L, et al. Systematic review: Glycomics as diagnostic markers for hepatocellular carcinoma. Aliment Pharmacol Ther 2024;59:23-38. [Crossref] [PubMed]
- Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243:213-21. [Crossref] [PubMed]