
Shrinking fat, healing liver: unlocking the metabolic dysfunction associated steatohepatitis puzzle
Metabolic dysfunction associated steatohepatitis (MASH) is a silent epidemic, hiding in the shadows of obesity, and often silently advancing towards the grave complication of cirrhosis. The distinction between MASH and simple hepatic steatosis, which is under the spectrum of metabolic dysfunction-associated steatotic liver disease (MASLD), is highly desired as it provides critical information about the prognosis of the patient. Noninvasive indicators, such as fibrosis-4 (FIB-4) index and nonalcoholic fatty liver disease (NAFLD) fibrosis score that includes conventional laboratory data and clinical parameters, are commonly used for screening patients with MASLD for MASH. The imaging methods, especially magnetic resonance imaging (MRI), are increasingly used for both quantifying liver fat and searching for clues of MASH as they are noninvasive and can evaluate the whole liver with no risks associated with invasive liver biopsy. MRI proton density fat fraction (MRI-PDFF) has proven effective for predicting histological steatosis grades (1). However, MRI-PDFF does not provide information about the presence of inflammation associated with steatohepatitis, or fibrosis. Magnetic resonance elastography (MRE) which evaluates the mechanical properties of the tissue is commonly used for this purpose. Research studies in MRE have demonstrated the effectiveness of this method in both discrimination of MASH from simple steatosis, and among fibrosis stages with high diagnostic overall accuracy (2-4). Currently, MRI-PDFF and MRE are recognized as the most accurate and reproducible methods for quantifying and grading hepatic steatosis, and detection and staging fibrosis, respectively in clinical practice.
Understanding the intricate relationship between fat depots and liver health has long been a puzzle for medical researchers. MRI also enables the evaluation of other fat depots including subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) in addition to liver fat. VAT is more metabolically active than SAT, and there is close association of VAT with metabolic syndrome, and thought to be mediated by its venous drainage into the portal vein. The association of abdominal obesity with MASLD has drawn attention to VAT. In a previous cross-sectional study that included bariatric cohorts as well as known MASLD patients, Idilman et al. observed significant correlations between VAT volume and histologic findings in the liver such as steatosis grade, NAFLD activity score, and fibrosis stage (5). The authors in the study concluded that the MRI-determined VAT volume can be used for predicting the presence of hepatic steatosis, MASH, and fibrosis in patients with known or suspected MASLD. In that study, however, no correlation was observed between SAT volume and histopathologic findings, nor between SAT and VAT volumes. However, recent studies propose different metabolic profile in two SAT anatomic compartments which are divided by Scarpa’s fascia as deep subcutaneous adipose tissue (dSAT) and superficial subcutaneous adipose tissue (sSAT). Brand et al. evaluated adult patients with a body mass index (BMI) ≥27 and observed positive correlations between hepatic fat, VAT, and dSAT but no correlation with sSAT (6). In the subgroup analyses, a positive correlation between hepatic fat and dSAT in men and a negative correlation between hepatic fat and sSAT in women was also observed (6). Tordjman et al. evaluated liver, VAT, dSAT, and sSAT biopsy specimens in 38 obese patients (7). In terms of morphology, adipocyte size, and macrophage accumulation, it was observed that the dSAT of obese individuals exhibits more similarity to VAT rather than sSAT (7). In this study, researchers also observed a higher number of macrophages in VAT and dSAT in patients with MASH and those with fibro-inflammation index ≥3 with no such changes in sSAT (7). These findings indicate close relationships between hepatic steatosis, and VAT and dSAT. Furthermore, VAT and dSAT volumes may be helpful in the prediction of hepatic inflammation and fibrosis which indicate MASH.
One of the unanswered issues in the follow-up evaluation of a MASH patient is prediction of disease progression. Stine et al. demonstrated a ≥30% relative decline in liver MRI-PDFF is associated with histologic response in patients with MASH (8). Gidener et al. evaluated serial MRE exams in patients with MASLD and concluded that MRE offers a promising noninvasive approach for longitudinally monitoring liver stiffness measurement (LSM) as well as a potential for estimating the risk of liver-related outcomes associated with MASLD (9).
In a groundbreaking study, a secondary analysis of data from the FLINT trial has shed new light on a significant link between reductions in adipose tissue volumes and hepatic histologic improvement in MASH patients (10). In this study, the authors included a total of 76 patients with MASLD and observed that VAT and dSAT volume changes were significantly correlated with histologic improvement in MASH. However, sSAT volume change was not found to have a significant correlation with histologic improvement. Multivariate analyses demonstrated that higher lobular inflammation at baseline, greater reduction in dSAT volume, and reduction in hepatic PDFF were associated with histologic improvement. These researchers also observed that the MRI model incorporating adipose tissue depots demonstrated significantly superior performance compared to the basic anthropometric model. The authors concluded that greater longitudinal reductions in dSAT volume and potentially VAT volumes showed greater hepatic histologic improvements, independent of reductions in hepatic PDFF. These findings suggest an association between changes in different fat depots with the alterations in the degree of hepatic steatosis and inflammation. Previous reports suggested that dSAT closely relates to VAT and that increased macrophages and inflammatory factors are found in dSAT in patients with MASH (7). The correlation of improvement with reduction in dSAT and central obesity i.e., VAT can prove valuable for the follow-up evaluation of MASLD patients. This would be particularly useful in clinical trials assessing therapeutic responses with estimation of VAT and dSAT volumes providing stronger evidence of changes occurring in response to treatment than just using anthropometric measurements. The study by Shen et al. also brings into focus multiple pathological processes that are ongoing elsewhere in the body concurrently or in tandem within the liver in MASLD and metabolic syndrome. MRI with new techniques play an important role in the assessment of fat depots, and relationship between them in MASLD as well as detection of MASH and evaluation of changes in the follow-up.
Adipose tissue volume increases by adipocyte hypertrophy (increase in the adipocyte size) and adipocyte hyperplasia (increase in the number of adipocytes). Adipocyte hypertrophy, in contrast to adipocyte hyperplasia is associated with development of inflammatory response, worsening insulin sensitivity, impaired glucose tolerance and lipid metabolism, and cardiovascular morbidity and associated outcomes independent of obesity (11). Increased adipocyte size is also associated with increased adipose tissue leptin secretion and reduced adiponectin levels. The SAT adipocytes are much larger than those in VAT suggesting an association with MASH through adipocyte induced inflammation. In another study of 68 patients with MASLD, Micu et al. (12) correlated histological changes in liver biopsy with blood tests for systemic inflammation (C-reactive protein, fibrinogen) and adipose tissue inflammation (leptin and adiponectin levels). They found that systemic and adipose tissue inflammation was significantly associated with histologic changes in MASH and concluded that determination of systemic inflammatory markers like C-reactive protein is predictive of progression of the disease.
In an exploratory study, Li et al. (13) performed 3D multiparametric MRI and MRE in 27 obese patients (BMI: 45±7 kg/m2; morbidly obese: 23/27; female: 23/27; age: 47±10 years) who had biopsies of liver and SAT, and found significant correlations between the mechanical properties of liver and subcutaneous fat, and their histological and biochemistry results. A model combining liver PDFF and subcutaneous fat stiffness had a slightly higher accuracy for diagnosing MASH than liver stiffness, although not statistically significant [area under the curve (AUC): 0.87 vs. 0.84, P=0.74]. The results indicate that metabolic dysfunction and obesity-induced systemic inflammation affects both adipose and liver tissue mechanical properties and, therefore, models utilizing mechanical biomarkers from adipose tissue may improve the diagnosis of steatohepatitis in obese patients. In a follow-up study, Li et al. found that shear stiffness and loss modulus of the liver improved following treatment with bariatric surgery. They also measured shear stiffness and loss modulus of the subcutaneous in the same cohort (unpublished data) and found that the SAT shear stiffness and loss modulus reduced during follow-up paralleling that of the improvements in liver mechanical parameters (Figure 1). These preliminary results highlight that MASLD is a systemic inflammatory disorder that involves liver and fat depots, and probably other organs. Imaging evaluation of more accessible tissues such as SAT, and probably more focused on dSAT may provide additional information in the pathogenesis and evaluation of treatment response in MASLD.
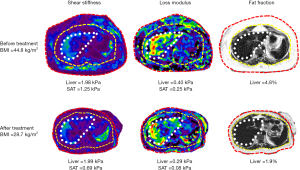
The studies by Shen et al. (10) and Li et al. (13,14) provide motivation for the utilization of MRI assessment, including advanced technologies like 3D vector MRE for adipose tissues. Quantitative evaluation of adipose tissue volumes and their mechanical properties could prove valuable for non-invasive assessment of MASLD and treatment response. Future research should focus on evaluating MASLD as a systemic disorder, encompassing the assessment of adipose tissue depots and other organs beyond the liver.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-569/coif). M.Y. received R01 grant (No. DK136731) and the Mayo Clinic and M.Y. have intellectual property and a financial interest related to magnetic resonance elastography (MRE) technology and she disclosed money paid to the Mayo Clinic for patents and royalties related to MRE technology and methods. M.Y. disclosed money paid to her for stock/stock options from Resoundant. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qadri S, Vartiainen E, Lahelma M, et al. Marked difference in liver fat measured by histology vs. magnetic resonance-proton density fat fraction: A meta-analysis. JHEP Rep 2023;6:100928. [Crossref] [PubMed]
- Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920-8. [Crossref] [PubMed]
- Costa-Silva L, Ferolla SM, Lima AS, et al. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur J Radiol 2018;98:82-9. [Crossref] [PubMed]
- Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26:1431-40. [Crossref] [PubMed]
- Idilman IS, Low HM, Gidener T, et al. Association between Visceral Adipose Tissue and Non-Alcoholic Steatohepatitis Histology in Patients with Known or Suspected Non-Alcoholic Fatty Liver Disease. J Clin Med 2021;10:2565. [Crossref] [PubMed]
- Brand T, van den Munckhof ICL, van der Graaf M, et al. Superficial vs Deep Subcutaneous Adipose Tissue: Sex-Specific Associations With Hepatic Steatosis and Metabolic Traits. J Clin Endocrinol Metab 2021;106:e3881-9. [Crossref] [PubMed]
- Tordjman J, Divoux A, Prifti E, et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol 2012;56:1152-8. [Crossref] [PubMed]
- Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2021;19:2274-2283.e5. [Crossref] [PubMed]
- Gidener T, Dierkhising RA, Mara KC, et al. Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in NAFLD. Hepatology 2023;77:268-74. [Crossref] [PubMed]
- Shen W, Middleton MS, Cunha GM, et al. Changes in abdominal adipose tissue depots assessed by MRI correlate with hepatic histologic improvement in non-alcoholic steatohepatitis. J Hepatol 2023;78:238-46. [Crossref] [PubMed]
- Ye RZ, Richard G, Gévry N, et al. Fat Cell Size: Measurement Methods, Pathophysiological Origins, and Relationships With Metabolic Dysregulations. Endocr Rev 2022;43:35-60. [Crossref] [PubMed]
- Micu ES, Amzolini AM, Barău Abu-Alhija A, et al. Systemic and adipose tissue inflammation in NASH: correlations with histopathological aspects. Rom J Morphol Embryol 2021;62:509-15. [Crossref] [PubMed]
- Li J, Obrzut M, Lu X, et al. Assessment of obesity-induced metabolic disorders in adipose tissue by multi-parametric MR Elastography (MRE). ISMRM & SMRT Virtual Conference & Exhibition; 2020.
- Li J, Allen AM, Shah VH, et al. Longitudinal Changes in MR Elastography-based Biomarkers in Obese Patients Treated with Bariatric Surgery. Clin Gastroenterol Hepatol 2023;21:220-222.e3. [Crossref] [PubMed]


