Living donor liver transplantation for hepatocellular carcinoma at the University of Tokyo Hospital
Introduction
Since the landmark study by Mazzaferro et al. (1) liver transplantation (LT) has become widely-accepted as an established treatment for patients with early stage hepatocellular carcinoma (HCC), defined as a single tumor smaller than 5 cm in diameter or up to three tumors smaller than 3 cm in diameter with no vascular invasion or extra-hepatic disease, Milan criteria. Milan criteria are also standard indication criteria for LT for HCC patients in Asian countries (2,3). However, in Asia where living donor liver transplantation (LDLT) is mainstay for LT, majority of centers use an expanded criteria without impairing the recipient outcomes (4,5). Unlike deceased donor liver transplantation (DDLT), LDLT is not limited by the restrictions imposed by the nationwide allocation system, and the indication for LDLT in patients with HCC often depends on institutional or case-by-case considerations, balancing the burden on the donor, the operative risk, and the overall survival benefit for the recipient (6). The main purpose of the present study is to present the results of LDLT for HCC patients with our extended criteria (Tokyo criteria, 5-5 rule) at the University of Tokyo Hospital.
During the review of our series, we also focused on additional two issues: (I) the association between the small-sized partial graft and HCC recurrence; and (II) the incidental intrahepatic cholangiocarcinoma (ICC) and combined hepatocellular carcinoma/cholangiocarcinoma (cHCC-CC) in liver explants. The former may include the possible supportive information to the recent controversy regarding LDLT versus DDLT for HCC patients in terms of the recurrence rate (7-9). The latter has become a topic in LT recently (10,11), and the accumulation of institutional reports will be of help in future studies in view of the rarity of this situation.
Methods
From January 1996 until the end of 2015, total of 573 patients, including 550 LDLT and 23 DDLT, underwent LT at the University of Tokyo. Among them, 139 patients have been indicated LDLT for the treatment of HCC, and were the subjects of the present study. All HCC recipients were donated from living donors. Preoperative diagnosis of HCC was based on dynamic multi-detector computed tomography (MDCT) performed within 1 month before LT in all cases. Lesions presenting with typical radiological characteristics of classical HCC, that is, lesions with enhancement in arterial phase and low density during portal phase, were diagnosed and counted as HCC. Essentially, we used the Milan criteria as a standard indication of LT for HCC, however, we allow the expanded criteria in LDLT setting, the detail of which is as follows; the number of tumor should be five or less, and the maximum diameter of the tumor should be 5 cm or less, without the distant metastasis nor the vascular invasion (Tokyo criteria, 5-5 rule). We do not use biomarkers, such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) in patient selection. As for the donor selection, an estimated graft volume to the recipient standard liver volume (SLV) ratio must be over 40% for LT at our institution, and the left liver was the first choice for the graft if it satisfied the lower limit. Otherwise, right liver procurement was indicated if the estimated right liver graft volume was less than 70% of the donor’s total liver volume, and a right lateral sector graft was used in selected cases. Details of the donor evaluation and graft selection are described elsewhere (12). The basic immunosuppression regimen comprised tacrolimus and steroid for all recipients, and the doses of each drug were gradually tapered over 6 months after LDLT. Our detailed postoperative recipient management including immunosuppression protocol is described elsewhere (13). We do not modify the immunosuppression for HCC recipients, and do not use m-TOR inhibitors nor adjuvant chemotherapies. All the patients were followed up at our department after LT according to the following protocol: monthly measurement of AFP and DCP, abdominal ultrasound performed every 3 months, and contrast-enhanced dynamic MDCT every 6 months. Recurrence was defined as emergence of radiological findings in MDCT compatible with typical HCC.
Statistical analysis
Categorical variables were expressed as number (%) and continuous variables were expressed as median with range. Categorical and continuous data were compared between groups using the chi-square, Fisher’s exact and Student’s t-test, or Mann-Whitney U test, as appropriate. Patient overall survival and recurrence-free survival was calculated using Kaplan-Meier with Log rank test. Univariate and multivariate logistic regression model was used to identify the predictors of HCC recurrence. Statistical calculations were performed with SPSS statistical software (version 22.0 for Windows, Chicago, IL, USA). P values less than 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Recipient demographics were summarized in Table 1. There were 107 males and 32 females, with a mean age of 55 (range, 37–67) years. Hepatitis C virus (HCV) positive cases comprised of 60% of the cohort. Model for end-stage liver disease (MELD) score was mean 13 (range, 2–34). Fifty-nine percent (82/139) had undergone loco-regional treatments for HCC before LT, including 13 cases with surgical resections, 27 with radiofrequency ablation, 21 with percutaneous ethanol injection therapy, and 64 with transarterial chemoembolization. There were no cases of intentional downstaging or bridging therapy in this cohort. The number and the size of the tumor were 2 (range, 0–14) and 2.0 (range, 0.5–8.0) cm in the preoperative radiologic evaluation, while those were 3 (range, 1–19) and 2.0 (range, 0.5–11.0) cm in the pathologic examination of the explants. In histologic examination of explants, vascular invasion was confirmed in 16/138 (11.6%) cases, and 67 (48%), 55 (40%), and 7 (5%) patients were with well-, moderately-, and poorly-differentiated HCC, respectively. Regarding the tumor burden, 119 cases were within Milan criteria, 14 cases were beyond Milan/within Tokyo criteria, and 6 cases were beyond Tokyo criteria in pretransplant radiologic evaluation, meaning 133/139 (95%) patients met the Tokyo criteria, but in the explants, 94 (68%) patients and 119 (86%) patients met Milan and Tokyo criteria, respectively.
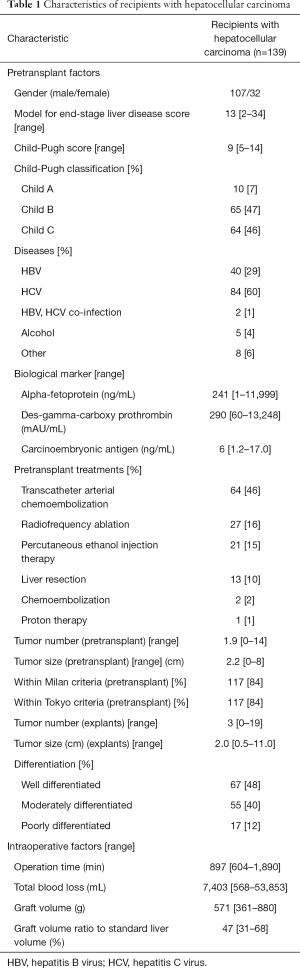
Full table
Recurrence-free and patient survival
The follow-up period was 148 (range, 0.8–1,366) months. The 1-, 5-, and 10-year recurrence-free and patient survival of all the cohort was 95%, 91%, and 91%, and 91%, 80%, and 78%, respectively in long-term. When the patients were divided into three groups, within Milan (n=119), beyond Milan and within Tokyo (n=14), and beyond Tokyo (n=6), both recurrence-free and patient survival of those beyond Tokyo were significantly impaired (P<0.001), while those of within Milan and beyond Milan/within Tokyo were comparable, as shown in Figure 1. Overall 11 (8%) patients experienced HCC recurrence. The 1-, 3-, and 5-year cumulative recurrence rate was 5%, 6%, and 6% for within Milan, 0%, 8%, and 8% for beyond Milan/within Tokyo, and 33%, 50%, and 50% for beyond Tokyo, respectively. There were totally 33 mortality cases in this cohort, and the death due to HCC recurrence were observed in nine patients.

Factors associated with HCC recurrence
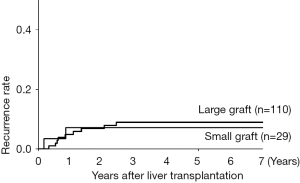
Risk factors associated with HCC recurrence were evaluated with univariate and multivariate analysis, which revealed that the high AFP value (≥400 ng/mL), the high DCP value (≥200 mAU/mL) and beyond the Tokyo criteria were significant predictors (Table 2). Focusing on the graft type and size, there was no relation between these graft characteristics and HCC recurrence. The 1-, 3-, and 5-year recurrence rates stratified by the graft size (the cutoff value: 40% to SLV of recipient) were comparable between the large graft (5%, 9%, and 9%) and the small graft (7%, 7%, and 7%), as shown in Figure 2 (P=0.83).
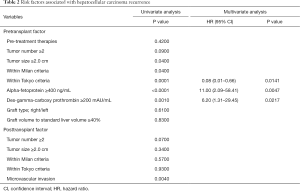
Full table
ICC and cHCC-CC
During this study period, we identified 3 patients (2%) with histological variant of primary liver cancer (one ICC and two cHCC-CC patients), all which had been incidentally confirmed in histologic investigation of explanted livers. Clinicopathological features of these cases were summarized in Table 3. There were one ICC in one patient and two cHCC-CCs in two patients. Two cHCC-CC nodules were misdiagnosed as HCC preoperatively, while an ICC nodule was not detected in preoperative evaluation but was found incidentally in pathology. All tumors were less than 2 cm in diameter, and were well to moderate in differentiation. In terms of tumor burden, all three were within Milan preoperatively, however, one patient exceeded even Tokyo criteria in histologic evaluation of the explant (seven nodules in total). Preoperative tumor markers including AFP, carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA 19-9) levels were elevated in two patients in each measurement, however, no supportive information for the preoperative diagnosis could be found due to the small number of cases and the slight increment of each marker. All patients were alive without recurrence with a follow-up period of 2 to 14 years.

Full table
Discussion
In this study, we reviewed our single-institution experience of LDLT for 139 HCC patients, the result of which seems acceptable in terms of the recurrence-free and patient survival.
The Japanese Ministry of Health, Labor, and Welfare considers LT indicated for patients with HCC within the Milan criteria, stating that (I) the decision of whether the patient satisfies the Milan criteria should be based on dynamic CT or MRI taken within 1 month before LT; (II) a pre-LT diagnosis of HCC means that the tumor demonstrates the classical pattern, low-high-low density, in dynamic contrast-enhanced CT; (III) and in cases that undergo loco-regional treatment prior to LT, at least a 3-month interval between the last treatment and LT is mandatory, and the indication should be based on images obtained within 1 month before LT (tumors judged as totally necrotic need not be counted). All Japanese institutions as well as our center, however, allow patients with tumor status that is beyond the Milan criteria to undergo LT according to the institution’s criteria as an uninsured treatment, provided that there is no contraindication such as macroscopic vascular invasion or extrahepatic metastases. Nevertheless, the latest report from Japanese (14) liver transplant registry reported that 1-, 3-, 5-, and 10-year survival after LDLT was 84%, 75%, 69%, and 61%, respectively, among 1,431 HCC recipients, which was comparable to those reported by the registry study in Europe (15) and USA (16).
HCC recurrence after LT remains a clinical issue regardless of the meticulous patient selection criteria (15). Based on the literature (17,18), HCC recurrence after LT uniformly occurs with an incidence of 10% to 20%. Well-recognized predictors of recurrence include tumor size and number, bilobar disease, tumor differentiation, presence of macro- and micro-vascular invasion and tumor satellites, and tumor-specific biomarkers such as AFP and DCP levels before LT (19-22). A meta-analysis (20) of 74 studies comprising 22,432 patients revealed that the diameter of the largest nodule or the total diameter of nodules was the best predictor of outcome, and that a total tumor size (sum of diameters) over 10 cm was associated with a four-fold higher risk of recurrence. Another meta-analysis (23) of 1,198 patients indicated that the presence of vascular invasion, poor differentiation, tumor diameter over 5 cm, and tumor status beyond the Milan criteria were independent risk factors for HCC recurrence. Unfortunately, these large studies do not include biomarkers such as AFP and DCP, however, there have been numerous reports (21,22,24), as well as the present study that report the significance of these biomarkers in predicting HCC recurrence after LT. Two other major Japanese centers, Kyoto (25) and Kyushu (26), indeed, include DCP value as their expanded criteria for HCC patients. The inclusion of biomarkers to the existing criteria based on the number and the size of tumor will be a critical issue in the future debate regarding the indication of LT for HCC. Another concern in the present study was that small size of the graft can affect the HCC recurrence after LT. Recently, Park et al. (8) reported that the recurrence is significantly higher in LDLT setting than in DDLT setting, but that the size of the graft in LDLT did not affect the recurrence. The same group also reported the possible association between parenchymal congestion of the partial graft and the recurrence (9). In the present study, we did not find any association between the graft size/type and HCC recurrence. The controversy whether LDLT imposes the increased risk of recurrence on patients is discussed in another review article on this focused issue, however, the present result may deny the link between graft regeneration and HCC recurrence.
Another aim of this study was to figure out the incidence and outcome of those with incidental ICC or cHCC-CC. Early reports of LT for ICC or cHCC-CC (27-30) had demonstrated dismal outcome with the reported 1-, 3-, and 5-year recurrence-free and patient survival around 70%, 50%, and 30%, 60%, 40%, and 20%, respectively. Accordingly, LT is in principle contraindicated for those diagnosed as having ICC or cHCC-CC preoperatively worldwide as well as in our institution. During the last decade, however, some authors (10,31-34) have published studies demonstrating the favorable outcome of those incidentally diagnosed as having cholangiocarcinoma in the histology of the liver explants. Recently, Sapisochin et al. (34) performed Spanish multicenter study of the outcome of patients with ICC and cHCC-CC on pathological examination of explants. They found that difference was found in 1-, 3- and 5-year actuarial survival rates between the ICC group (n=27) and the HCC control group (n=54) (78%, 66%, and 51% vs. 100%, 98%, and 93%; P<0.001), while no difference was observed between the cHCC-CC group (n=15) and the HCC control group (n=30) (93%, 78%, and 78% vs. 97%, 86%, and 86%; P=0.9). In the subgroup analysis for those with solitary incidental ICC or cHCC-CC less than 2 cm in diameter, there was no difference in patient survival when compared to the HCC control group. These studies (31,32,34-38) were summarized in Table 4. Based on the recent reports as well as our own experience, the indication of LT for preoperatively diagnosed cHCC-CC could be the same with HCC. Regarding the ICC, patients diagnosed preoperatively with a solitary tumor less than 2 cm in diameter could be a possible candidate for LT. Yet, further accumulation of records and a large multicenter study are expected to settle the indication of LT for these tumors, especially for ICC.

Full table
In conclusion, we presented our experience of LDLT for HCC. The issues of the expansion of indication, LDLT vs. DDLT for HCC, and LT for ICC are still left to be investigated in future studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the research ethics committee and institutional review board of the University of Tokyo (No. 2185) and written informed consent was obtained from all patients.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Ng KK, Lo CM, Chan SC, et al. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary Pancreat Sci 2010;17:548-54. [Crossref] [PubMed]
- Lee SG, Moon DB, Shin H, et al. Living donor liver transplantation for hepatocellular carcinoma: current status in Korea. Transplant Proc 2012;44:520-2. [Crossref] [PubMed]
- Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer 2014;3:108-18. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kokudo N. Living-donor vs deceased-donor liver transplantation for patients with hepatocellular carcinoma. World J Hepatol 2014;6:626-31. [Crossref] [PubMed]
- Chan SC. Section 2. Small-for-size liver graft and hepatocellular carcinoma recurrence. Transplantation 2014;97 Suppl 8:S7-S10. [Crossref] [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014;97:71-7. [Crossref] [PubMed]
- Suh SW, Lee JM, You T, et al. Hepatic venous congestion in living donor grafts in liver transplantation: is there an effect on hepatocellular carcinoma recurrence? Liver Transpl 2014;20:784-90. [Crossref] [PubMed]
- Hashimoto K, Miller CM. Liver transplantation for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2015;22:138-43. [Crossref] [PubMed]
- Sapisochín G, Fernández de Sevilla E, Echeverri J, et al. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol 2015;7:2396-403. [Crossref] [PubMed]
- Kokudo N, Sugawara Y, Imamura H, et al. Tailoring the type of donor hepatectomy for adult living donor liver transplantation. Am J Transplant 2005;5:1694-703. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kanako J, et al. Low Platelet Counts and Prolonged Prothrombin Time Early After Operation Predict the 90 Days Morbidity and Mortality in Living-donor Liver Transplantation. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Umeshita K, Inomata Y, Furukawa H, et al. Liver transplantation in Japan -Registry by the Japanese Liver Transplantation Society. Hepatol Res 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol 2012;57:675-88. [Crossref] [PubMed]
- Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation 2013;95:755-60. [Crossref] [PubMed]
- Welker MW, Bechstein WO, Zeuzem S, et al. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int 2013;26:109-18. [Crossref] [PubMed]
- Todo S, Furukawa H, Tada M, et al. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2007;13:S48-54. [Crossref] [PubMed]
- Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-40. [Crossref] [PubMed]
- Germani G, Gurusamy K, Garcovich M, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl 2011;17 Suppl 2:S58-66. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5.
- Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832-8. [Crossref] [PubMed]
- Sotiropoulos GC, Molmenti EP, Lösch C, et al. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res 2007;12:527-34. [PubMed]
- Soriano A, Varona A, Gianchandani R, et al. Selection of patients with hepatocellular carcinoma for liver transplantation: Past and future. World J Hepatol 2016;8:58-68. [Crossref] [PubMed]
- Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053-60. [Crossref] [PubMed]
- Shirabe K, Taketomi A, Morita K, et al. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant 2011;25:E491-8. [Crossref] [PubMed]
- Patkowski W, Stankiewicz R, Grąt M, et al. Poor outcomes after liver transplantation in patients with incidental cholangiocarcinoma irrespective of tumor localization. Transplant Proc 2014;46:2774-6. [Crossref] [PubMed]
- Castellano-Megías VM, Ibarrola-de Andrés C, Colina-Ruizdelgado F. Pathological aspects of so called "hilar cholangiocarcinoma". World J Gastrointest Oncol 2013;5:159-70. [Crossref] [PubMed]
- Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 2004;239:265-71. [Crossref] [PubMed]
- Shimoda M, Farmer DG, Colquhoun SD, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl 2001;7:1023-33. [Crossref] [PubMed]
- Facciuto ME, Singh MK, Lubezky N, et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation 2015;99:151-7. [Crossref] [PubMed]
- Takahashi K, Obeid J, Burmeister CS, et al. Intrahepatic Cholangiocarcinoma in the Liver Explant After Liver Transplantation: Histological Differentiation and Prognosis. Ann Transplant 2016;21:208-15. [Crossref] [PubMed]
- Sapisochin G, Fidelman N, Roberts JP, et al. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl 2011;17:934-42. [Crossref] [PubMed]
- Sapisochin G, de Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg 2014;259:944-52. [Crossref] [PubMed]
- Maganty K, Levi D, Moon J, et al. Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma: outcome after liver transplantation. Dig Dis Sci 2010;55:3597-601. [Crossref] [PubMed]
- Chan AC, Lo CM, Ng IO, et al. Liver transplantation for combined hepatocellular cholangiocarcinoma. Asian J Surg 2007;30:143-6. [Crossref] [PubMed]
- Itoh S, Ikegami T, Yoshizumi T, et al. Long-term outcome of living-donor liver transplantation for combined hepatocellular-cholangiocarcinoma. Anticancer Res 2015;35:2475-6. [PubMed]
- Serra V, Tarantino G, Guidetti C, et al. Incidental Intra-Hepatic Cholangiocarcinoma and Hepatocholangiocarcinoma in Liver Transplantation: A Single-Center Experience. Transplant Proc 2016;48:366-9. [Crossref] [PubMed]


