
Prevalence, patterns, risk factors and outcomes of peritoneal metastases after laparoscopic hepatectomy for hepatocellular carcinoma: a multicenter study from China
Highlight box
Key findings
• Peritoneal metastases (PM) prevalence after curative laparoscopic hepatectomy (LH) is 2.9% in this multi-center retrospective observational study.
• Laparoscopic surgery would not augment PM risk if undertaken by experienced surgeons.
What is known and what is new?
• The prevalence, patterns and independent risk factors of PM were identified for hepatocellular carcinoma (HCC) patients after LH.
• Aggressive surgery for recurrent PM might benefit long-term prognosis for HCC.
What is the implication, and what should change now?
• Increased caution is required for surgeons during the initial learning phase when performing LH for HCC.
Introduction
Peritoneal metastases (PM) are recognized at laparoscopy or autopsy at a prevalence of 3% to 16% (1,2). PM are indicators of a dismal prognosis for patients with hepatocellular carcinoma (HCC) (3). Systemic chemotherapy is first-line treatment for PM in HCC (4), achieving a median survival of 2.1–12.5 months which is much lower than that for patients without PM (5). Several studies have reported that hyperthermic intraperitoneal chemotherapy (HIPEC) and aggressive peritoneal metastasectomy can prolong median overall survival (OS) to 46.7 months in patients with relapsed HCC (6-9). However, only 36.1% of these patients are eligible for curative re-excision (6,10), and the prognosis is strongly influenced by the extent of peritoneal lesions. Unfortunately, the detection methods and serum biomarkers employed for evaluation of PM recurrence fail to recognize them early and robust predictive markers for PM are lacking. Hence, early prediction of PM status in HCC is crucial to devise more individualized management to prolong survival.
Laparoscopic hepatectomy (LH) has been applied to treat HCC. The number of HCC patients undergoing LH has grown considerably (11). Studies have demonstrated that laparoscopic procedures carry an inspiring long-term prognosis compared with that using open hepatectomy (OH) (12-14). However, there are persistent doubts regarding the risk of PM because viable tumor cells may contaminate laparoscopic wounds via direct transfer from laparoscopic instruments or by aerosolization of malignant cells liberated into the peritoneal cavity during pneumoperitoneum (15). To date, only limited studies on PM following LH have been reported. However, due to the small number of patients and single-center data recruitment, the authors did not draw a convincing conclusion as to whether LH augmented the PM risk (16). Some researchers have argued that tumor diameter >5 cm, microvascular invasion (MVI), and positive margins are potential risk factors for PM during open surgery (6,17). Nevertheless, the prevalence, risk factors and molecular mechanism underlying PM after LH have not been elucidated. Furthermore, recognition of small peritoneal nodules at an early stage of HCC is difficult because of the unsatisfactory discrimination abilities of imaging devices. Therefore, the prevalence and risk factors for PM after LH merits further study so as to take preventive measures during resection as well as to plan the postoperative follow-up program.
Using a large, multicenter cohort, we investigated the prevalence, patterns, risk factors, treatment, and long-term outcomes associated with PM in HCC after LH. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-506/rc).
Methods
Patient characteristics
Using a multicenter dataset, 2,138 consecutive patients who underwent curative LH for HCC between August 2010 to December 2016 in seven Chinese hospitals were identified. “Curative LH” was defined as complete removal of all lesions with a clear margin (R0 resection). The inclusion criteria were: (I) curative liver resection; (II) primary HCC diagnosed pathologically with absence of distant metastases; (III) no macroscopic vascular invasion or tumor rupture; (IV) no previous treatment for HCC; (V) precise follow-up information and data on prognostic variables. This research was carried out in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Review Board of Tongji Hospital (Wuhan, China) (TJ-IRB20210935). Written informed consent for clinical research of the data generated during therapy was obtained from all enrolled patients.
Preoperative assessment and surgical procedures
Preoperative assessment was conducted 1–2 days before surgery. The resectability of liver lesions was defined according to complete imaging survey and preoperative liver function. Child-Pugh grade C was identified as an absolute contraindication to surgery. The type of hepatectomy was decided mainly by integrated consideration of the tumor, liver status, and the retention rate of indocyanine green, as described previously (18). Perioperative management was (in general) standardized and consistent at all participating centers.
Definition and management of PM
Follow-up details were obtained from outpatient review, medical records, and telephone interviews. In general, routine workup incorporated detection of liver function and tumor markers, ultrasonography, enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) once every 3 months in the first year, and every 4–6 months thereafter. Positron emission tomography (PET; using 18F-FDG), and PET-CT were undertaken if necessary. Recognition of recurrent PM was based on positive findings of imaging examination and incorporation of data for enhanced CT and/or MRI (19,20). Further imaging examinations for recurrence screening were done if relevant laboratory abnormalities and symptoms were identified.
“PM recurrence” was defined as intra-abdominal local recurrence with tumor growth in the peritoneum, microscopic tumor growth in the peritoneum or ascites containing cancer cells (21). We classified patients into those without PM (“No-PM”) and those with PM. Further analysis of resectable PM and unresectable PM was carried out. Appropriate management strategies for relapsed disease were determined based on recurrence patterns and performance status. The criteria for patients receiving aggressive surgery were as follows: having resectable PM and without compromising essential anatomic structures such as major vasculature, manageable or resectable intrahepatic recurrences (IHRs), good Eastern Cooperative Oncology Group (ECOG) performance (0–1), Child’s A liver disease, and adequate heart and renal function (6,10). In general, complete peritoneal metastasectomy [cytoreductive surgery (CRS)] plus HIPEC or concurrent liver resection was considered to be potentially curative resection. Palliative therapy incorporated repeat resection of peritoneal lesions as much as possible and/or local therapy in synchronous liver lesions.
Statistical analyses
Categorical variables are expressed as numbers and percentages. Continuous covariates are presented as the median and interquartile range (IQR). Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test (two-tailed χ2 test). Comparisons between continuous variables were conducted using the Mann-Whitney U-test. Subsequently, X-tile (Yale University, New Haven, CT, USA) was used to identify the optimal cutoff of alpha-fetoprotein (AFP), diameter, and the Peritoneal Cancer Index (PCI). Age, sex, tumor size, tumor number and Barcelona Clinic Liver Cancer (BCLC) stage were taken as covariates, and 1:1 matching between the LH and OH groups was conducted within a caliper value of 0.02. Univariable and multivariable logistic regression analyses were undertaken to identify the potential risk factors of PM. OS was calculated using the Kaplan-Meier method. Subgroup analyses were done in patients who developed PM in HCC. The difficulty of LH was graded by the classification by Kawaguchi et al. (22). Univariate and multivariate Cox regression analyses were used to evaluate the predictors associated with long-term survival in patients with PM. R 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses. P<0.05 (two-sided) was considered significant.
Results
Clinicopathological characteristics at the initial resection
Recruitment pathway of eligible HCC patients and work flow is presented in Figure 1. Table 1 presents the clinicopathological characteristics of the enrolled LH patients. Of the 2,138 patients who were enrolled in the LH group, 1,826 (85.4%) were male and the median age of patients with primary HCC was 52.1 (IQR, 39.1–68.8) years. We found that 1,909 patients (89.3%) had chronic infection with the hepatitis-B virus and 672 (31.4%) patients had a high AFP level (≥400 ng/mL). Most individuals had an early-stage tumor (BCLC stage 0–A: n=1,997, 93.4%) and well-compensated liver function (Child-Pugh class A: n=2,049, 95.8%). Median tumor diameter was 4.2 (IQR, 2.5–5.0) cm and 239 (11.2%) patients had multiple lesions. A lesion <2 cm from a major blood vessel was noted for 30.1% patients (n=644). At the time of surgery, all patients underwent curative LH with a R0 resection. Postoperative pathology revealed a group of patients with satellite foci (n=338, 15.8%) and intratumor necrosis (n=216, 10.1%). Most masses were well-to-moderately differentiated (n=1,179, 55.1%); a subset of HCC lesions had MVI (n=663, 31.0%). There were 1,490 HCC patients enrolled in the OH group, while 1,075 cases were divided into the OH patients and analyzed subsequently after 1:1 propensity score matching (PSM). The baseline and clinicopathologic characteristics before and after PSM were presented in Table 2. The distributions of propensity scores before and after matching were summarized in Figure S1.
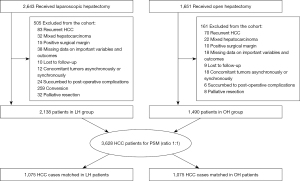
Table 1
| Variables | Value (n=2,138) |
|---|---|
| Age (years) | 52.1 [39.1–68.8] |
| Gender (male) | 1,826 (85.4) |
| BMI (kg/m2) | |
| <18.5 | 190 (8.9) |
| 18.5–24.9 | 1,584 (74.1) |
| ≥25.0 | 364 (17.0) |
| Diabetes (yes) | 210 (9.8) |
| HBV infection (yes) | 1,909 (89.3) |
| HCV infection (yes) | 66 (3.1) |
| AFP (≥400 ng/mL) | 672 (31.4) |
| NLR | 2.3 [1.4–2.6] |
| Platelet (×103/µL) | 147.9 [104.0–190.0] |
| BCLC stage | |
| 0 | 426 (19.9) |
| A | 1,571 (73.5) |
| B | 141 (6.6) |
| Child-Pugh grade (A) | 2,049 (95.8) |
| Tumor diameter, cm | 4.2 [2.5–5.0] |
| Tumor number (multiple) | 239 (11.2) |
| Lesions <2 cm from major blood vessela (yes) | 644 (30.1) |
| Liver cirrhosis (yes) | 1,342 (62.7) |
| Portal hypertension (yes) | 694 (32.5) |
| Surgical difficultyb | |
| Low | 1,067 (49.9) |
| Intermediate | 398 (18.6) |
| High | 673 (31.5) |
| Resection margin ≤1 cm | 588 (27.5) |
| Blood loss (mL) | 200 [80–430] |
| Type of hepatectomy (non-anatomical) | 1,496 (70.0) |
| Extent of hepatectomy (major) | 297 (13.9) |
| Satellite nodules (yes) | 338 (15.8) |
| Intratumor necrosis (yes) | 216 (10.1) |
| MVI (yes) | 663 (31.0) |
| Tumor differentiation (poor) | 959 (44.9) |
Data are presented as median [interquartile range] or n (%). a, major hepatic vein and inferior vena cava; b, difficulty scoring system for laparoscopic liver resection proposed by Kawaguchi et al. HCC, hepatocellular carcinoma; LH, laparoscopic hepatectomy; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; NLR, neutrophil to lymphocyte ratio; BCLC stage, Barcelona Clinic Liver Cancer stage; MVI, microvascular invasion.
Table 2
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| LH (n=2,138) | OH (n=1,490) | P value | LH (n=1,075) | OH (n=1,075) | P value | ||
| Age (years) | 52.1 [39.1–68.8] | 52.4 [39.3–68.9] | 0.075 | 52.2 [39.3–67.8] | 52.7 [39.6–68.5] | 0.556 | |
| Gender (male) | 1,826 (85.4) | 1,268 (85.1) | 0.856 | 900 (83.7) | 892 (83.0) | 0.527 | |
| BMI (kg/m2) | 0.213 | 0.102 | |||||
| <18.5 | 190 (8.9) | 148 (9.9) | 99 (9.2) | 103 (9.6) | |||
| 18.5–24.9 | 1,584 (74.1) | 1,086 (72.9) | 791 (73.6) | 795 (73.9) | |||
| ≥25.0 | 364 (17.0) | 256 (17.2) | 185 (17.2) | 177 (16.5) | |||
| Diabetes (yes) | 210 (9.8) | 136 (9.1) | 0.302 | 103 (9.6) | 102 (9.5) | 0.221 | |
| HBV infection (yes) | 1,909 (89.3) | 1,325 (88.9) | 0.151 | 947 (88.1) | 952 (88.6) | 0.321 | |
| HCV infection (yes) | 66 (3.1) | 43 (2.9) | 0.081 | 25 (2.3) | 30 (2.8) | 0.375 | |
| AFP (≥400 ng/mL) | 672 (31.4) | 486 (32.6) | 0.137 | 361 (33.6) | 353 (32.8) | 0.210 | |
| NLR | 2.3 [1.4–2.6] | 2.2 [1.3–2.5] | 0.412 | 2.2 [1.5–2.7) | 2.2 [1.4–2.65] | 0.301 | |
| Platelet (×103/µL) | 147.9 [104.0–190.0] | 148.1 [103.6–191.2] | 0.321 | 147.2 [103.5–191.2] | 148.3 [104.6–190.5] | 0.710 | |
| Child-Pugh grade (A) | 2,049 (95.8) | 1,411 (94.7) | 0.564 | 1,022 (95.1) | 1,019 (94.8) | 0.687 | |
| BCLC stage | 0.015 | 0.375 | |||||
| 0 | 426 (19.9) | 282 (18.9) | 226 (21.0) | 215 (20.0) | |||
| A | 1571 (73.5) | 1,107 (74.3) | 777 (72.3) | 788 (73.3) | |||
| B | 141 (6.6) | 101 (6.8) | 72 (6.7) | 72 (6.7) | |||
| Tumor diameter, cm | 4.2 [2.5–5.0] | 4.10 [2.1–5.5] | 4.0 [2.5–5.0] | 4.0 [2.5–5.0] | 0.653 | ||
| Tumor number (multiple) | 239 (11.2) | 162 (10.9) | 113 (10.5) | 112 (10.4) | 0.0923 | ||
| Lesions <2 cm from major blood vessel (yes)a | 644 (30.1) | 465 (31.2) | 0.042 | 323 (30.0) | 329 (30.6) | 0.071 | |
| Liver cirrhosis (yes) | 1,342 (62.7) | 1,050 (70.5) | 0.021 | 769 (71.5) | 765 (71.2) | 0.068 | |
| Portal hypertension (yes) | 694 (32.5) | 580 (38.9) | 0.024 | 354 (32.9) | 347 (32.3) | 0.072 | |
| Resection margin ≤1 cm | 588 (27.5) | 337 (22.6) | 0.216 | 259 (24.1) | 268 (24.9) | 0.196 | |
| Blood loss (mL) | 200 [80–430] | 210 [130–450] | 0.012 | 200 [110–460] | 200 [110–450] | 0.056 | |
| Extent of hepatectomy (major) | 297 (13.9) | 246 (16.5) | 0.008 | 156 (14.5) | 159 (14.8) | 0.092 | |
| Satellite nodules (yes) | 338 (15.8) | 224 (15.0) | 0.191 | 161 (15.0) | 167 (15.5) | 0.635 | |
| Intratumor necrosis (yes) | 216 (10.1) | 185 (12.4) | 0.035 | 118 (11.0) | 112 (10.4) | 0.321 | |
| MVI (yes) | 663 (31.0) | 456 (30.6) | 0.073 | 323 (30.0) | 325 (30.2) | 0.231 | |
| Tumor differentiation (poor) | 959 (44.9) | 672 (45.1) | 0.091 | 474 (44.1) | 483 (44.9) | 0.065 | |
Data are presented as median [interquartile range] or n (%). a, major hepatic vein and inferior vena cava. PSM, propensity score matching; LH, laparoscopic hepatectomy; OH, open hepatectomy; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; NLR, neutrophil to lymphocyte ratio; BCLC, Barcelona Clinic Liver Cancer; MVI, microvascular invasion.
Prevalence, patterns, and distribution of PM after LH and OH
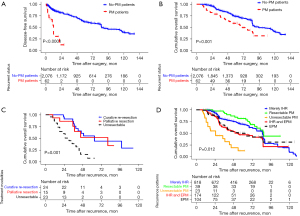
During a median follow-up of 67.0 months, a total of 1,158 (54.2%, LH group) and 752 (50.5%, OH group) patients had HCC recurrence, with median DFS times of 11 months (IQR, 6–17) and 11 months (IQR, 5–16), respectively. There was no significant difference between the two groups regarding the recurrence patterns, DFS values or treatments, except of LH having a higher risk of IHR compared with OH (70.6% vs. 69.1%, P=0.013), which was balanced after PSM (Table S1); 2.9% (62/2,138) of HCC patients developed PM after LH in our multicenter cohort, which was lower than 4.0% (59/1,490) in the OH patients (P=0.041). Nevertheless, after PSM, the PM of 3.3% in the LH group was close to the 3.5% in the OH group (P=0.906) (Table 3). Almost all of the PM occurred within 2 years after surgery (Figure 2A). Among PM patients in the LH group, 26 (41.9%) had PM alone, 34 (54.9%) had PM coupled with IHR and 2 (3.2%) had PM with extraperitoneal metastases (EPM). The most common site of PM was the omentum (n=29, 46.7%). About one-quarter of patients had a single lesion (n=15, 24.2%). The median number of PM was 2.0 (IQR, 1.0–3.0) and median total diameter of PM was 5.6 (IQR, 2.7–8.0) cm. The patterns, and distribution of PM had no significant difference between the two groups neither before nor after PSM (P>0.05). Interestingly, in the LH group, the PM prevalence during the early period [2010–2013] was significantly higher than that during the later period [2014–2016] (5.1% vs. 2.6%, P=0.016), although the LH during the later period seemed to more difficult than the early period, e.g., larger tumors, more likely located in the posterosuperior segment of the liver, closer to major blood vessels than tumors, more anatomical resection and higher surgical difficulty during the later period (Table 3, Table S2). While, the PM incidence after OH was similar between the two periods (3.9% vs. 4.0%, P=0.148).
Table 3
| Variables | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| LH | OH | P value | LH | OH | P value | ||
| PM incidence | 62/2,138 (2.9) | 59/1,490 (4.0) | 0.041 | 36/1,075 (3.3) | 38/1,075 (3.5) | 0.906 | |
| Early period [2010–2013] | 15/295 (5.1) | 39/990 (3.9) | 0.042 | 7/150 (4.7) | 25/675 (3.7) | 0.083 | |
| Later period [2014–2016] | 47/1,843 (2.6) | 20/500 (4.0) | 0.098 | 29/925 (3.1) | 13/400 (3.3) | 0.094 | |
| Recurrent patterns | |||||||
| PM only | 26 (41.9) | 23 (39.0) | 0.262 | 16 (44.4) | 17 (44.7) | 0.725 | |
| PM coupled with IHR | 34 (54.9) | 33 (55.9) | 0.523 | 19 (52.8) | 20 (52.6) | 0.109 | |
| PM with synchronous extraperitoneal metastasis | 2 (3.2) | 3 (5.1) | 0.303 | 1 (2.8) | 1 (2.7) | 0.117 | |
| Distribution | |||||||
| Location of peritoneal lesions | |||||||
| Omentum | 29 (46.7) | 27 (45.7) | 0.414 | 16 (44.5) | 17 (44.7) | 0.535 | |
| Posterior peritoneum | 14 (22.6) | 13 (22.0) | 0.865 | 8 (22.2) | 8 (21.1) | 0.107 | |
| Anterior peritoneum/abdominal wall | 12 (19.5) | 13 (22.0) | 0.657 | 7 (19.4) | 8 (21.1) | 0.802 | |
| Combination | 7 (11.3) | 6 (10.2) | 0.582 | 5 (13.8) | 5 (13.1) | 0.904 | |
| Posterior peritoneum with omental nodules | 4 (6.5) | 3 (5.1) | 0.694 | 3 (8.3) | 3 (7.8) | 0.186 | |
| Posterior peritoneum with abdominal wall | 3 (4.8) | 3 (5.1) | 0.532 | 2 (5.5) | 2 (5.3) | 0.232 | |
| No. of lesions | |||||||
| Single peritoneal lesion | 15 (24.2) | 15 (25.4) | 0.412 | 9 (25.0) | 10 (26.3) | 0.613 | |
| Multiple peritoneal lesions | 47 (75.8) | 44 (74.6) | 0.517 | 27 (75.0) | 28 (73.7) | 0.501 | |
| Median No. of lesions | 2.0 [1.0–3.0] | 2.3 [1.1–3.9] | 0.044 | 2.2 [1.0–3.6] | 2.2 [1.0–3.6] | 0.122 | |
| Total diameter of peritoneal lesion, cm | 5.6 [2.7–8.0] | 5.5 [2.4–8.2] | 0.856 | 5.6 [2.7–8.5] | 5.6 [2.5–8.1] | 0.182 | |
| DFS (mon) | 8 [2–20] | 7 [2–19] | 0.636 | 7 [2–19] | 7 [2–19] | 0.165 | |
Data are presented as median [interquartile range] or n (%). PM, peritoneal metastasis; LH, laparoscopic hepatectomy; OH, open hepatectomy; PSM, propensity score matching; IHR, intrahepatic recurrence; DFS, disease-free survival.
Risk factors for PM after LH
Univariate analyses using logistic regression demonstrated that the BCLC stage, tumor diameter, tumor number, intratumor necrosis, type of hepatectomy, lesion <2 cm from a major blood vessel, MVI, and tumor differentiation were significant factors that increased the likelihood of PM after curative LH (P<0.05 for all). Tumor diameter >5 cm [odds ratio, 2.383, 95% confidence interval (CI): 2.077–4.659], non-anatomical hepatectomy (odds ratio, 3.486, 95% CI: 1.004–6.189), lesion <2 cm from a major blood vessel (odds ratio, 3.959, 95% CI: 1.730–9.062) and MVI (odds ratio, 1.863, 95% CI: 1.215–5.196) remained independent risk factors of PM for HCC patients after curative LH by multivariate analysis (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P valuea | OR (95% CI) | P value | ||
| Sex (male vs. female) | 1.162 (0.548–2.467) | 0.695 | |||
| BMI (≥25.0 vs. <25.0 kg/m2) | 1.805 (0.807–4.037) | 0.151 | |||
| Diabetes (yes vs. no) | 1.374 (0.645–2.929) | 0.410 | |||
| HBV infection (yes vs. no) | 1.373 (0.416–4.527) | 0.603 | |||
| AFP (≥400 vs. <400 ng/mL) | 1.297 (0.768–2.189) | 0.331 | |||
| BCLC stage (B vs. 0/A) | 3.839 (2.290–6.433) | <0.001 | 1.015 (0.025–2.018) | 0.978 | |
| Child-Pugh grade (B vs. A) | 2.080 (0.813–5.325) | 0.127 | |||
| Tumor diameter (>5 vs. ≤5 cm) | 3.467 (2.068–5.737) | <0.001 | 2.383 (2.077–4.659) | 0.003 | |
| Tumor number (solitary vs. multiple) | 6.317 (3.741–10.668) | <0.001 | 1.127 (0.915–2.138) | 0.075 | |
| Liver cirrhosis (yes vs. no) | 1.115 (0.626–1.985) | 0.526 | |||
| Portal hypertension (yes vs. no) | 0.917 (0.530–1.568) | 0.857 | |||
| Extent of hepatectomy (major vs. minor) | 1.350 (0.695–2.621) | 0.375 | |||
| Satellite nodules (yes vs. no) | 1.583 (0.863–2.905) | 0.138 | |||
| Intratumor necrosis (yes vs. no) | 2.047 (1.050–3.993) | 0.035 | 4.222 (0.964–9.120) | 0.863 | |
| Type of hepatectomy (non-anatomical vs. anatomical) |
4.847 (2.456–7.575) | 0.016 | 3.486 (1.004–6.189) | 0.029 | |
| Surgical difficulty (high vs. low/intermediate) | 6.235 (2.632–13.536) | 0.025 | 2.121 (0.969–3.698) | 0.635 | |
| Lesions <2 cm from major blood vessel (yes vs. no)b |
2.925 (1.758–4.866) | <0.001 | 3.959 (1.730–9.062) | 0.002 | |
| MVI (yes vs. no) | 3.583 (1.863–6.574) | 0.008 | 1.863 (1.215–5.196) | 0.032 | |
| Tumor differentiation (poor vs. well/moderate) | 2.322 (1.197–4.192) | 0.047 | 2.706 (1.815–4.033) | 0.065 | |
a, variables with a P value <0.05 in univariate analysis were subjected to multivariate logistic regression analyses using forward stepwise variable selection; b, major hepatic vein and inferior vena cava. PM, peritoneal metastasis; HCC, hepatocellular carcinoma; LH, laparoscopic hepatectomy; OR, odds ratio; CI, confidence interval; BMI, body mass index; HBV, hepatitis B virus; AFP, α-fetoprotein; BCLC stage, Barcelona Clinic Liver Cancer stage; MVI, microvascular invasion.
Treatments and outcomes of PM after LH
Of the 62 patients who developed PM in LH group, 24 patients (38.7%) underwent potentially curative treatments, which incorporated curative repeat resection of peritoneal lesions (n=16) or curative surgery for PM and IHR (n=8); 15 patients (24.2%) underwent palliative resection of peritoneal lesions; another 23 patients (37.1%) who had advanced tumor staging and/or poor liver function had nonoperative management (unresectable) (Table S3). Together, 39 (62.9%) patients underwent CRS. All PM patients who underwent re-excision experienced HIPEC therapy after surgery. The complications for CRS/HIPEC were graded according to the National Cancer Institute CTCAE v5.0. Grade 2 or 3 adverse events were observed in 4 patients as shown in Table S4. There were no grade 4 adverse events or perioperative fatalities in this study. With a median follow-up of 39 months in PM patients, 36 deaths from PM were observed; two cases with PM and synchronous EPM had OS of 13 months (bone metastases) and 6 months (lung metastases), respectively. No-PM patients had a significantly better long-term outcome than PM patients, and 5-year OS was 72.0% and 55.0%, respectively (P<0.001) (Figure 2B). However, 5-year OS for resectable PM (curative/palliative) patients was significantly longer (65.0%) compared with that of unresectable patients (9.0%) (P=0.001) (Figure 2C). Figure 2D illustrates OS for various groups of recurrences in comparison with resectable PM and unresectable PM. Patients with resectable PM had similar OS compared with that of patients with IHR alone (P=0.235) but it was significantly longer than that of patients with other types of EPM (P=0.012). It is worth mentioning that PM occurring within 1 year had a significant worse prognosis than late recurrence patients (≥1 year), as the Figure S2 depicted. Furthermore, we accessed the risk factors of prognosis for PM patients after LH. And we found PM coupled with IHR or PM with synchronous EPM (hazard ratio, 4.713, 95% CI: 1.278–9.639, P=0.032), PCI ≥8 (hazard ratio, 1.746, 95% CI: 1.017–3.250, P=0.021) and palliative/unresectable treatment mode (hazard ratio, 0.361, 95% CI: 0.151–0.602, P=0.035) were independent risk factors of the long-term outcomes for PM patients after LH. While time to recurrence ≥1 year was a favorable factor in univariate Cox regression analysis for PM patients, but not independent prognostic factor in multivariate Cox regression analyses (Table S5).
Discussion
Accumulating evidence suggests PM to be a significant cause of mortality after curative LH for HCC. Although PM prevalence of 3–18% has been reported in HCC patients from autopsy evidence (23), such metastases may be overlooked due to the limited sensitivity of examination methods, which could severely limit the prognosis of HCC patients. Investigations on the prevalence, patterns, risk factors, treatment, and outcomes of PM after LH for HCC are lacking. Our study represents the first multi-center study on the postoperative PM following LH, providing convincing evidence that LH had no effect on increasing PM prevalence. Furthermore, aggressive surgery for recurrent PM may improve the prognosis of HCC patients.
Studies have demonstrated that laparoscopic surgery has comparable long-term outcomes compared with open surgery (24-26), and this is also confirmed by our present findings (Figure S3). However, concerns remain among many surgeons about PM risk, especially during the initial learning phase. Some researchers have considered that viable cancer cells might contaminate the abdominal cavity via direct transfer from laparoscopic instruments. Further, the pneumoperitoneum may promote seeding of cancer cells into the peritoneal cavity (27). Nevertheless, recent studies have found that laparoscopic surgery and open surgery have a comparable prevalence of local recurrence and peritoneal dissemination in cervical and rectal cancer (28-30). The PM prevalence for HCC patients after LH and OH in the present study were 2.9% and 4.0%, respectively. And the difference was not significant after PSM, as with the patterns, timing, and treatments of recurrence between the two approaches. The PM rates of 2.9% after LH in the present study was comparable with that of 3.0% in a nationwide study from 1,222 cases underwent OH in Korea (6). Interestingly, the PM prevalence of 5.1% in the early period of the LH group was significantly higher than 2.6% in the later period of the LH group. Studies have demonstrated individual surgeons during the learning curve to be the dominant risk factors of poor outcomes (31,32). Similarly, the prevalence of local recurrence in laparoscopic surgery for rectal cancer was shown to decrease with increasing experience of surgeons, especially in those with advanced disease (33). Therefore, it is reasonable to suggest that LH had no effect on increasing the prevalence of PM in HCC patients for experienced surgeons. However, close supervision during surgery by highly experienced surgeons, selection of patients with a low risk of recurrence, and careful intraoperative manipulation should be advocated for inexperienced surgeons to reduce the risk of PM in HCC patients.
In this large multicenter retrospective study, tumor diameter >5 cm, non-anatomical hepatectomy, MVI, and a lesion <2 cm from a major blood vessel were identified as independent risk factors for PM. Larger tumor diameter (>5 cm) and MVI have been reported to be associated with PM (6,17). However, whether non-anatomical hepatectomy is associated with PM in HCC patients is not known. Kaibori and colleagues found non-anatomical resection to be associated significantly with extrahepatic recurrence (especially local dissemination) after hepatic resection (34). Studies have illustrated that non-anatomical resection does not remove small, subclinical metastases in the residual liver segment (35,36). Intrahepatic microscopic metastases disseminating via the portal-vein branches along the residual liver segment are the main reasons for tumor recurrence in HCC patients (incorporating intrahepatic and extrahepatic recurrence) (37,38). Moreover, a non-anatomical resection procedure would augment the risk of tumor cells becoming detached and spreading to the free peritoneal cavity, as depicted in gastric cancer (39,40). In addition, non-anatomical resection could contribute to extrahepatic recurrence via circulating tumor cells with epithelial-to-mesenchymal transition in the residual segment (41,42), which increases the risk of abdominal metastases significantly.
We found that tumor location <2 cm from a major blood vessel during LH was associated significantly with PM development. During LH, the proximity of a lesion to a major blood vessel will increase the difficulty of surgery, particularly for inexperienced surgeons. Moreover, greater intraoperative blood loss and an increased risk of tumor recurrence has been observed for a tumor <2 cm from a major blood vessel because massive intraoperative bleeding can increase the risk for intraoperative tumor spillage to the abdominal cavity and hematogenous spread (43), especially in patients who have non-anatomical resection (44). Presumably, a tumor close to a major blood vessel would carry a greater risk of extra-tumoral MVI and potential distant hematogenous metastases (45,46). Notably, high degree of surgical difficulty was associated with higher risk of PM in the univariate analysis, but this was not an independent predictor in the multivariable analysis. Generally, the higher difficulty score of LH usually accompanied by prolonged operation time and increased risk of bleeding, which is associated with worse outcomes (44).
The rationale of surgical treatment for PM remains controversial and standard treatment is not available (8). Treatment guidelines for PM in HCC in Japan and Western countries recommend systemic chemotherapy (4). In general, PM in HCC are rarely suitable for curative repeat surgical excision, and most patients have PM with synchronous IHR or distant metastases, which was also demonstrated in our cohort. Nevertheless, studies have suggested that surgical removal of peritoneal lesions as much as possible might improve the prognosis of selected patients. In Japan, one study which investigated the largest number of PM patients so far reported 5-year OS of 92 patients who underwent peritoneal metastasectomy plus HIPEC to be 36.0% (17). A multicenter international study demonstrated that aggressive surgical management of PM generated favorable long-term survival (47). In the present study, 5-year OS of PM patients was 55.0%, which is higher than that reported previously (6.0–49.4%) (6,8,47). This difference might be attributed to more aggressive intervention in our study: 62.9% (39/62) of PM patients underwent potentially curative or palliative treatments in our cohort, which reduced the tumor burden in PM patients significantly. Hence, we recommend removing as much PM as possible to improve the long-term prognosis of PM patients.
Our study benefited from a large cohort and a multicenter-study design. Nevertheless, it had four main limitations. First, owing to complex anatomy and the limitations of imaging, recognition of small recurrent lesions was challenging and evaluation of follow-up outcomes was influenced. Second, though there is a unified treatment plan in HCC patients, there would inevitably be differences between centers (e.g., surgical plan, surgeon’s experience, postoperative management), which might influence data reproducibility. Nevertheless, our results are reflective of real-world conditions and make them generalizable to some degree. Third, the retrospective, non-randomized nature of our study represents its biggest limitation because it entails a selection bias. Hence, further prospective multicenter studies are warranted to verify our conclusions. Fourth, comparative investigations in patients who did not undergo peritoneal metastasectomy were lacking, and inclusion of such work is planned in our future studies.
Conclusions
We identified in a multicenter study, for the first time, PM prevalence after curative LH to be 2.9%. We revealed that tumor diameter >5 cm, non-anatomical resection, presence of MVI, and a lesion <2 cm from a major blood vessel to be independent risk factors of PM after curative LH for HCC. Laparoscopic surgery would not augment PM risk if undertaken by experienced surgeons. Nevertheless, increased caution is required for surgeons lacking laparoscopic experience when treating PM. For peritoneal lesions, aggressive surgery may improve the prognosis of HCC patients significantly.
Acknowledgments
The authors thank all the surgeons and patients who participated in this study.
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-506/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-506/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-506/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was carried out in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent for data use was obtained from all patients. The study protocol was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, within Huazhong University of Science and Technology (TJ-IRB20210935).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kudo M, Izumi N, Kubo S, et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res 2020;50:15-46. [Crossref] [PubMed]
- Kasai Y, Tamaki A, Kakita H. Surgical treatment of primary hepatocellular carcinoma. Geka Shinryo 1977;19:46-54.
- Roussel E, Bubenheim M, Le Treut YP, et al. Peritoneal Carcinomatosis Risk and Long-Term Survival Following Hepatectomy for Spontaneous Hepatocellular Carcinoma Rupture: Results of a Multicenter French Study (FRENCH-AFC). Ann Surg Oncol 2020;27:3383-92. [Crossref] [PubMed]
- Kudo M, Trevisani F, Abou-Alfa GK, et al. Hepatocellular Carcinoma: Therapeutic Guidelines and Medical Treatment. Liver Cancer 2016;6:16-26. [Crossref] [PubMed]
- Lin CC, Liang HP, Lee HS, et al. Clinical manifestations and survival of hepatocellular carcinoma patients with peritoneal metastasis. J Gastroenterol Hepatol 2009;24:815-20. [Crossref] [PubMed]
- Kow AW, Kwon CH, Song S, et al. Risk factors of peritoneal recurrence and outcome of resected peritoneal recurrence after liver resection in hepatocellular carcinoma: review of 1222 cases of hepatectomy in a tertiary institution. Ann Surg Oncol 2012;19:2246-55. [Crossref] [PubMed]
- Yeh CN, Chen MF, Jeng LB. Resection of peritoneal implantation from hepatocellular carcinoma. Ann Surg Oncol 2002;9:863-8. [Crossref] [PubMed]
- Kow AW, Kwon CH, Song S, et al. Clinicopathological factors and long-term outcome comparing between lung and peritoneal metastasectomy after hepatectomy for hepatocellular carcinoma in a tertiary institution. Surgery 2015;157:645-53. [Crossref] [PubMed]
- Ji ZH, An SL, Li XB, et al. Long-term progression-free survival of hepatocellular carcinoma with synchronous diffuse peritoneal metastasis treated by CRS+HIPEC: A case report and literature review. Medicine (Baltimore) 2019;98:e14628. [Crossref] [PubMed]
- Hung KC, Yang KL, Huang GC, et al. Cytoreduction surgery and hyperthermic intraperitoneal chemotherapy for treating advanced peritoneal metastases of hepatocellular carcinoma. Pleura Peritoneum 2020;5:20190030. [Crossref] [PubMed]
- Yoon YI, Kim KH, Kang SH, et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients With Cirrhosis: A Propensity Score Matched Analysis. Ann Surg 2017;265:856-63. [Crossref] [PubMed]
- Xiang L, Li J, Chen J, et al. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg 2016;103:1895-901. [Crossref] [PubMed]
- Zhu P, Liao W, Zhang WG, et al. A Prospective Study Using Propensity Score Matching to Compare Long-term Survival Outcomes After Robotic-assisted, Laparoscopic, or Open Liver Resection for Patients With BCLC Stage 0-A Hepatocellular Carcinoma. Ann Surg 2023;277:e103-11. [Crossref] [PubMed]
- Dumronggittigule W, Han HS, Komoltri C, et al. Laparoscopic versus open hepatectomy for large hepatocellular carcinoma: a single center propensity-score-matching comparative analysis of perioperative outcomes and long-term survival. Surg Endosc 2023;37:2997-3009. [Crossref] [PubMed]
- Bouvy ND, Marquet RL, Jeekel H, et al. Impact of gas(less) laparoscopy and laparotomy on peritoneal tumor growth and abdominal wall metastases. Ann Surg 1996;224:694-700; discussion 700-1. [Crossref] [PubMed]
- Zhang H, Liu F, Wen N, et al. Patterns, timing, and predictors of recurrence after laparoscopic liver resection for hepatocellular carcinoma: results from a high-volume HPB center. Surg Endosc 2022;36:1215-23. [Crossref] [PubMed]
- Iida H, Tani M, Aihara T, et al. New metastasectomy criteria for peritoneal metastasis of hepatocellular carcinoma: A study of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2020;27:673-81. [Crossref] [PubMed]
- Chen Y, Zhao J, Zhang Z, et al. Construction and Validation of a Nomogram for Predicting the Risk of Deep Vein Thrombosis in Hepatocellular Carcinoma Patients After Laparoscopic Hepatectomy: A Retrospective Study. J Hepatocell Carcinoma 2021;8:783-94. [Crossref] [PubMed]
- An H, Lee EYP, Chiu K, et al. The emerging roles of functional imaging in ovarian cancer with peritoneal carcinomatosis. Clin Radiol 2018;73:597-609. [Crossref] [PubMed]
- Honma Y, Terauchi T, Tateishi U, et al. Imaging peritoneal metastasis of gastric cancer with (18)F-fluorothymidine positron emission tomography/computed tomography: a proof-of-concept study. Br J Radiol 2018;91:20180259. [Crossref] [PubMed]
- Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012;99:699-705. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Ikai I, Arii S, Ichida T, et al. Report of the 16th follow-up survey of primary liver cancer. Hepatol Res 2005;32:163-72. [Crossref] [PubMed]
- Meguro M, Mizuguchi T, Kawamoto M, et al. Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery 2015;158:573-87. [Crossref] [PubMed]
- Hirokawa F, Hayashi M, Miyamoto Y, et al. Short- and long-term outcomes of laparoscopic versus open hepatectomy for small malignant liver tumors: a single-center experience. Surg Endosc 2015;29:458-65. [Crossref] [PubMed]
- Hyung WJ, Yang HK, Park YK, et al. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol 2020;38:3304-13. [Crossref] [PubMed]
- Chen S, Zheng Y, Tong L, et al. Laparoendoscopic Single-site Radical Hysterectomy with Vaginal Closure and without Uterine Manipulator for FIGO IB1 Cervical Cancer. J Minim Invasive Gynecol 2020;27:1471-2. [Crossref] [PubMed]
- Bogani G, Ghezzi F, Chiva L, et al. Patterns of recurrence after laparoscopic versus open abdominal radical hysterectomy in patients with cervical cancer: a propensity-matched analysis. Int J Gynecol Cancer 2020;30:987-92. [Crossref] [PubMed]
- Ohtani H, Tamamori Y, Arimoto Y, et al. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer 2012;3:49-57. [Crossref] [PubMed]
- Chen K, Cao G, Chen B, et al. Laparoscopic versus open surgery for rectal cancer: A meta-analysis of classic randomized controlled trials and high-quality Nonrandomized Studies in the last 5 years. Int J Surg 2017;39:1-10. [Crossref] [PubMed]
- Teo JY, Kam JH, Chan CY, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr 2015;4:379-90. [Crossref] [PubMed]
- Goh BK, Chan CY, Wong JS, et al. Factors associated with and outcomes of open conversion after laparoscopic minor hepatectomy: initial experience at a single institution. Surg Endosc 2015;29:2636-42. [Crossref] [PubMed]
- Kim CH, Kim HJ, Huh JW, et al. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol 2014;110:989-96. [Crossref] [PubMed]
- Kaibori M, Kon M, Kitawaki T, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2017;24:616-26. [Crossref] [PubMed]
- Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 2005;242:252-9. [Crossref] [PubMed]
- Ochiai T, Sonoyama T, Kikuchi S, et al. Anatomic wide hepatectomy for treatment of hepatocellular carcinoma. J Cancer Res Clin Oncol 2007;133:563-9. [Crossref] [PubMed]
- Shindoh J, Hasegawa K, Inoue Y, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford) 2013;15:31-9. [Crossref] [PubMed]
- Zhao H, Chen C, Gu S, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: A propensity score matching analysis. J Gastroenterol Hepatol 2017;32:870-8. [Crossref] [PubMed]
- Na JU, Lee SI, Noh SM. The single incision laparoscopic intragastric wedge resection of gastric submucosal tumor. J Gastric Cancer 2011;11:225-9. [Crossref] [PubMed]
- Chen ZX, Chen JP, Chen Z, et al. Treatment of cancerous ascites and radical gastrectomy with intraperitoneal hyperthermic double-distilled water and cis-diaminodichloro-platinum perfusion. World J Gastroenterol 1997;3:246-8. [Crossref] [PubMed]
- Zhang Q, Rong Y, Yi K, et al. Circulating tumor cells in hepatocellular carcinoma: single-cell based analysis, preclinical models, and clinical applications. Theranostics 2020;10:12060-71. [Crossref] [PubMed]
- Qi LN, Ma L, Chen YY, et al. Outcomes of anatomical versus non-anatomical resection for hepatocellular carcinoma according to circulating tumour-cell status. Ann Med 2020;52:21-31. [Crossref] [PubMed]
- Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009;249:617-23. [Crossref] [PubMed]
- Halls MC, Cipriani F, Berardi G, et al. Conversion for Unfavorable Intraoperative Events Results in Significantly Worse Outcomes During Laparoscopic Liver Resection: Lessons Learned From a Multicenter Review of 2861 Cases. Ann Surg 2018;268:1051-7. [Crossref] [PubMed]
- Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol 2019;26:1474-93. [Crossref] [PubMed]
- Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. [Crossref] [PubMed]
- Mehta S, Schwarz L, Spiliotis J, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol 2018;44:1786-92. [Crossref] [PubMed]


