
Existing and emerging biomarkers in hepatocellular carcinoma: relevance in staging, determination of minimal residual disease, and monitoring treatment response: a narrative review
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common cancer overall, and the second most common cause of cancer specific mortality in the world (1). For many years there was a steady increase in incidence of HCC in the US (6–7 cases per 100,000). Most recently, there has been a plateau in most demographics believed to be due in large part to the success of antiviral therapies for hepatitis C (2). Surveillance protocols for patients at high risk of HCC (hepatitis B, C, D and cirrhosis), only diagnose one third of HCC cases and greater than half of cases present with Barcelona Clinic Liver Cancer (BCLC) B or higher disease, highlighting the need for better surveillance and more effective systemic therapies (3).
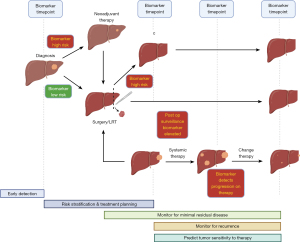
For years, sorafenib, a protein kinase inhibitor was the mainstay of systemic therapy for advanced HCC and offered only a 3-month overall survival (OS) benefit (4). The most significant development comes from the landmark IMbrave 150 trial examining atezolizumab [programmed death ligand 1 (PD-L1) inhibitor] and bevacizumab (vascular endothelial growth factor inhibitor) in unresectable HCC demonstrating improved overall and progression free survival compared to sorafenib. However, with these recent advancements in systemic therapy for unresectable and metastatic HCC, translation into the neoadjuvant and adjuvant space remains limited by the paucity of biomarkers to stage, monitor response to therapy and determine minimal residual disease (Figure 1). Alpha-fetoprotein (AFP) remains by far the most prevalent biomarker throughout five major phases of HCC management: (I) early detection through screening of high risk patients; (II) as part of a risk stratification program to decide on optimal therapy (e.g., locoregional therapy, resection, transplant etc.); (III) detection of minimal residual disease after resection who may benefit from adjuvant therapy; (IV) part of post-resection surveillance strategy to monitor for recurrence; and (V) to aid in selection of therapeutic agents to which tumors’ may be more sensitive. However, its use and widespread applicability is significantly limited as large multicenter studies of HCC have demonstrated that as high as 40–60% of all HCC patients are AFP negative, thus decreasing its utility as a standalone biomarker (5,6).
Next generation sequencing (NGS) made significant advances in characterizing the genetics of HCC. Though the mutational profile of HCC has demonstrated a paucity of druggable targets as well as significant inter- and intra-tumor heterogeneity (7). Thus, the advancement of adjuvant therapy for HCC has been limited. Additionally, genetic biomarker utilization has been limited by the lack of available tumor tissue in HCC largely due to the fact that its diagnosis can be made with only imaging and AFP level. The promise of liquid biopsy [the detection and molecular analysis of cancer related products in the blood stream, including but not limited to tumor nucleic acids, extracellular vesicles and circulating tumor cells (CTCs)] hopes to address this challenge by obviating the need for tissue or invasive procedures beyond blood draws (8).
In 2022, the newly published BCLC staging model incorporates biomarkers. Additionally, the update incorporates the application of immunotherapy for patients with advanced disease, further emphasizing the need for simple and accurate methods to predict and monitor treatment response (9).
In this review we aim to examine the existing literature describing biomarkers in HCC for staging and prognosis, determination of minimal residual disease as well as uses for monitoring response to systemic therapy. Our aim is to highlight the most well described clinical applications and most promising biomarkers, focusing especially on surveillance post resection or transplantation for HCC. We present this article in accordance with the Narrative Review reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-526/rc).
Methods
Both PubMed and Embase databases were searched for English language manuscripts using the keywords; hepatocellular carcinoma, biomarker, minimal residual disease, surveillance, prognosis, staging, AFP, liquid biopsy, treatment response, adjuvant, immunotherapy. Relevant studies were reviewed by all authors for appropriateness for inclusion (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 9/14–9/15/2022 |
| Databases and other sources searched | PubMed, Embase |
| Search terms used | Hepatocellular carcinoma, biomarker, minimal residual disease, surveillance, prognosis, staging, AFP, liquid biopsy, treatment response, adjuvant, immunotherapy |
| Timeframe | No limitation |
| Inclusion and exclusion criteria | Inclusion: all English language publications |
| Selection process | Search by DAD and PW. Studies reviewed by all authors for appropriate inclusion |
| Any additional considerations, if applicable | None |
AFP, alpha-fetoprotein.
Staging
An optimal staging system should easily provide accurate prognostic information from the patient’s history, imaging, and tissue, and should utilize testing that is widely available to guide therapeutic management decisions for patients with HCC. Additionally, in HCC, patient prognosis is in large part driven by underlying liver disease, creating a distinct challenge for the development of accurate prognostic staging systems in contrast to other cancer sites. This creates a two-fold need for biomarker application, for staging of existing liver disease, as well as staging of the tumor itself. Multiple existing staging systems including the BCLC, and Cancer of the Liver Italian Program (CLIP) staging system has incorporated biomarkers of liver function including bilirubin, international normalized ratio (INR), and creatinine, as well as the tumor marker AFP. Arguably the most widely used staging system for HCC, the BCLC criteria is also likely the most holistic, including factors on patients over all functional status, liver specific functional status, tumor criteria, and treatment efficacy, providing accurate prognostic information to guide therapy. The guidelines were recently updated in 2022 and have now incorporated the recommendation for use of the albumin-bilirubin (ALBI) score (a formula incorporating only albumin and bilirubin) to aid stratification of liver dysfunction, as well as the use of AFP to identify patients at high risk for recurrence.
AFP
By far the most widely used biomarker that can be elevated in HCC is AFP [reference range, 0–9.2 ng/mL (10)], a protein produced in the fetal liver that declines to a low level by the age of one (11). It is widely available in many in-hospital laboratories, as well as reference labs, and is relatively inexpensive with a price around $33 (12). Most data regarding sensitivity and specificity using AFP comes from screening data, and are 46–59% and 87–93% using a cut-off of 20 ng/mL, respectively (13,14). However, there are significant differences in AFP expression both by etiology of HCC (viral vs. non-viral), as well as the histologic subtype (Table 2). Macrotrabecular HCC, a more aggressive histologic subtype, has been associated with significantly higher AFP expression levels compared to other subtypes (21). Despite this variation, the majority of staging systems have now incorporated it as a biomarker to augment prognostic power (Figure 2).
Table 2
| % of HCC expressing elevated AFP | Reference | |
|---|---|---|
| Viral | ||
| HBV | 88.1% (10 ng/mL cut-off) | (15-17) |
| 79.6% (11.62 ng/mL cut-off) | ||
| 49.4% (20 ng/mL cut-off) | ||
| HCV | 11.6% (10 ng/mL cut-off) | (17-19) |
| 17.6% (5 ng/mL cut-off) | ||
| 36.4% (20 ng/mL cut-off) | ||
| HBV/HCV co-infection | 85.7% (5 ng/mL cut-off) | (19) |
| HBV/HCV negative | 20.5% (5 ng/mL cut-off) | (19) |
| Non-viral | ||
| Alcoholic liver disease | 12.8% (20 ng/mL cut-off) | (17,20) |
| 65.7% (20 ng/mL cut-off) | ||
| Non-alcoholic steatohepatitis | 47.0% (20 ng/mL cut-off) | (20) |
AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus.

The most recent staging system to incorporate AFP has been the BCLC system, published recently in 2022. While the recent update does highlight previous studies that associate elevated AFP with poor prognosis, it also acknowledges a lack of sufficiently studied cutoffs, and its incorporation into the model itself remains vague, stating that utilization of the model for prognosis is to be “refined by AFP, ALBI score, Child-Pugh, MELD” (9,22,23). Additionally, the update is in agreement with findings from multiple groups that elevated AFP is associated with higher recurrence risk, and recommends a cut off of 1,000 ng/dL as an exclusion criterion for patients with BCLC-B disease, being considered for extended liver transplant criteria (24-27). The wide variation in the use of AFP in staging regarding both cutoffs, as well as the subsets of patients in which it is applied within a staging system highlight the lack of predictive power of its use as a biomarker, and emphasize the need for more sensitive and specific biomarkers to augment staging prognostication.
ALBI score
Previously the BCLC staging system utilized the Child-Turcotte Pugh (CTP) system for functional hepatic reserve, however, the accessibility of certain factors such as ascites, and encephalopathy can be limited, greatly reducing utility (28). The development of the ALBI score in 2015, utilizing a composite formula of albumin and bilirubin demonstrated its simplicity and utility in further delineating prognostic subgroups among CTP classes (29). An international multicenter validation across BCLC stages in 2016 demonstrated that the ALBI score was a significant predictor of OS after surgical resection (P<0.001), transarterial chemoembolization (TACE) (P<0.001) and systemic therapy only with sorafenib (P<0.001), and was independent of BCLC stage (30). Similar to AFP, the updated BCLC criteria includes reference to the ALBI score only in a general sense to refine prognosis, and there are no specific guidelines regarding decision-making based on ALBI grades.
Bilirubin, albumin, AFP-L3, AFP, DCP (BALAD) score
In an effort to provide a more objective staging system, the BALAD score was created and validated in a Japanese cohort in 2006 (31). It is based solely on 5 serum biomarkers: bilirubin, albumin, lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3), AFP, and des-gamma-carboxy prothrombin (DCP). Further refinement was done by utilizing continuous forms of each variable rather than splitting each variable by cutoffs as in the original BALAD score, and has been validated in cohorts from both the United Kingdom, and most recently in North America (32,33).
Prognosis
AFP
When AFP is applied in the preoperative setting for prognostication, the data is mixed regarding its accuracy. In a retrospective analysis of 1,182 patients undergoing attempted curative resection in Hong Kong, the median OS was 38.4 months for patients with AFP >400 ng/mL, compared to 132.9 months in patients with an AFP <20 ng/mL (P<0.001). Additionally, in patients undergoing transplant evaluation for HCC, elevated AFP, particularly an AFP of >1,000 ng/mL has been utilized as an exclusion criterion by multiple high-volume centers due to associated with significantly worse outcomes after transplant (34,35). While some studies have found no difference in recurrence free and OS in patients undergoing either curative hepatectomy or thermal ablation for HCC (36,37), very high levels of AFP (>1,000 ng/mL) appear to have a clear association with poor prognostic factors including larger tumors, microvascular invasion, tumor multiplicity, as well as disease-free and OS (25,38). Reasons for these contrasting results are likely due to a multitude of factors, including different patient cohorts between Asian, European and North American cohorts, not only in race and ethnicity, but in etiology of HCC (39).
AFP-L3
AFP can be separated into three glycoforms based on its affinity with lens culinaris agglutinin (LCA), and are named as such, L1-3. AFP-L3% [reference range, 0–9.9 ng/mL, L3% <10% (40)] is the fraction that is bound by the LCA, and has been suggested as a biomarker more specific to a malignant source of AFP, as opposed to that produced in chronic hepatitis and cirrhosis (41,42). This test costs approximately $128 self-pay and is available through centralized specialty laboratories (12). In the previously mentioned review of patients undergoing curative hepatectomy or ablation, while AFP level was not associated with outcomes, elevated AFP-L3% >10% was associated with decreased survival in both groups (P=0.0171) (31). Additionally, a meta-analysis of 4,465 patients from fifteen studies examining pre-treatment AFP-L3% demonstrated that not only was it associated with both disease-free survival (DFS) [hazard ratio (HR): 1.80, 95% CI: 1.49–2.17) and OS (HR: 1.65, 95% CI: 1.45–1.89), it more importantly maintained this significance in patients with low AFP concentrations (HR: 2.53 95% CI: 1.09–5.89; HR: 1.96, 95% CI: 1.24–3.10; respectively) (43).
DCP
DCP [reference range, 0–7.5 ng/mL (44)] is an abnormal form of the prothrombin protein lacking carboxylated glutamic acid residues, due to an HCC cell specific lack of vitamin K-dependent gamma-glutamyl carboxylase (45). Similar to AFP-L3%, it is also available through reference laboratories for approximately $128 (12). Serum levels have been associated with more aggressive tumor specific factors including tumor differentiation, vascular invasion, intrahepatic metastasis, TNM stage, and size (46). In a systematic review and meta-analysis of six retrospective studies of 943 patients treated with trans-arterial chemotherapy, a lower DCP level was associated with improved OS (HR: 0.653, 95% CI: 0.444–0.960) (47). Interestingly, this effect was not seen in an analysis of 801 patients undergoing either hepatectomy or locoregional ablation with curative intent. In the 345 patients undergoing hepatectomy, there was no association between DCP levels and survival. However, in the 456 patients who underwent locoregional ablation, between AFP, AFP-L3 and DCP, DCP had the strongest association with patient survival (P=0.0004) (31).
Determination of minimal residual disease (MRD)and post-operative surveillance
The term MRD has been long associated with liquid malignancies and represents the small number of cancer cells remaining in the body after treatment. The advent of more sensitive techniques for detecting MRD have made the idea of detecting minimal residual disease in solid cancers more feasible. This is especially important given the recent promising results from the IM Brave 050 trial of atezolizumab plus bevacizumab in the adjuvant setting for patients at high risk of recurrence met their primary endpoint for recurrence-free survival on interim analysis (48). In patients at risk of residual disease after resection, particularly in HCC with microvascular invasion, it has been proposed that a wider resection margin may improve survival by decreasing the chances of MRD, however, data comes from only one randomized controlled trial (RCT), and it remains more likely that outcomes are more reflective of tumor biology rather than margin status (49). A meta-analysis of 7 studies including 1,932 patients undergoing surgical resection found improved 5-year OS in patients with a margin >1 cm compared with a sub cm margin [odds ratio (OR): 1.76, 95% CI: 1.20–2.59] (50). In addition to margin status alone, it has also been proposed that anatomic resection as compared to non-anatomic resection, may result in improved outcomes by removal of tumor-bearing portal territories. A systematic review and meta-analysis of 9,444 patients suggested improved 5-year disease-free and OS, although no included studies were prospective (51). However, liver resections are limited both by the size and function of the liver remnant, as well as the association of the tumor with critical structures which may preclude the clearing of all microscopic disease, making increasing the margin size in many patients impossible. The presence of microvascular invasion, is only detectable post operatively from surgical specimens, limiting its preoperative detection for prognostication and treatment planning (52). Improved methods of detection of the presence of minimal residual disease after resection would represent a significant improvement in prognostication which now relies mainly on histopathology. Additionally, while these markers may predict the presence of MRD after resection by way of the surrogate, microvascular invasion, this still does not address ongoing monitoring and detection. Emerging data for adjuvant therapy (48), could serve to select patients at high risk for early recurrence (larger tumors, higher AFP, tumor multiplicity etc.), while other biomarkers and imaging can still be used to monitor for later recurrence (53), Therefore, the development and validation of circulating tumor markers that can work in tandem to enhance existing surveillance imaging strategies including MRI and CT to allow for the early detection of recurrent disease after resection to guide subsequent therapy.
Role of protein markers in the detection of MRD
Given the presence of microvascular invasion on final pathology has been associated with early recurrence and poor prognosis after resection, its detection preoperatively has been proposed as a surrogate for MRD. Prediction of microvascular invasion from preoperative imaging is poor, however, studies of protein biomarkers have shown some ability in predicting microvascular invasion. In patients undergoing liver transplant for HCC, an AFP level greater than 100 was significantly associated with presence of microvascular invasion on pathology (OR: 5.0, 95% CI: 1.4–18.1), and presence of microvascular invasion was associated with risk of early recurrence and death (54). These findings were reproduced in a retrospective review of 1,153 patients undergoing liver resection, both total tumor volume and AFP were found to be independent risk factors associated with the presence of microvascular invasion (55). Given the lack of accuracy from imaging prediction of microvascular invasion, the combination of AFP with imaging was combined to improve preoperative prediction of microvascular invasion and improved the accuracy relative to other existing risk scores (AUC =0.800 in testing cohort n=125) (56). Elevated serum levels of DCP, also known as PIVKA-II (protein induced by vitamin K absence/antagonism-II) were similarly strongly associated with the presence of microvascular invasion on pathology (HR: 3.5, 95% CI: 1.08–11.8). The presence of high DCP tissue expression on immunostaining of surgical specimens was strongly associated with the presence of microvascular invasion as well (P<0.001) (57). In order to strengthen the preoperative prediction power of available tumor characteristics, including AFP, a prediction risk scoring system was devised using an artificial neural network utilizing serum AFP, number of tumor nodules, size of the largest nodule and total tumor volume to predict the presence of microvascular invasion in 250 patients with cirrhosis undergoing resection for HCC. The study found a positive predictive value of 91.9%, but has yet to be validated in an external cohort (58). However, different disease states in the absence of malignancy including chronic liver disease and hepatitis can inherently cause production of the proteins in question making external prospective validation of these risk scores necessary prior to widespread adoption.
Liquid biopsy
The lack of a sensitive and specific circulating biomarker in HCC has been a major limiting factor in monitoring of post operative MRD as well as decision making regarding choice and initiation of systemic therapy (8). The detection of cell-free DNA (cfDNA) and RNA (cfRNA) allows for a whole host of potential biomarkers, from measuring total amount, mutations, integrity, epigenetic changes. Within this circulating nucleic acid, is the subset of circulating tumor DNA (ctDNA) which represents mutations known to be present in the primary tumor. Existing comprehensive commercial tests include but are not limited to Grail Galleri ($949) (59), FoundationOne Liquid Cdx ($5,800) (60) and Guardant360 ($5,000) (61) and while they have the potential to provide a significant amount of information, they do carry a significantly increased cost compared with standard protein biomarkers therefore as new sequencing based tests are developed, a balance must be maintained with depth of sequencing, as this is a main driver of cost and often past a certain depth does not provide added benefit (62). CtDNA has already showed promise in HCC in early detection in the screening phase, a systematic review and meta-analysis demonstrated improved sensitivity (76.0% vs. 47.8%) and specificity (92.0% vs. 84.0%) when combined with AFP, compared to AFP alone (63,64). One of the earlier applications as a demonstration of the potential power of cfDNA, analyzed serum samples in patients with hepatitis C related HCC and compared it with healthy controls, finding a significantly increased amount of cfDNA in sera from HCC patients, which was superior to AFP and DCP in discriminating between healthy control and HCC patients (65). A systematic review and meta-analysis of 8 studies of ctDNA in Asian patients with HCC found that the presence of pre-treatment ctDNA was independently associated with decreased DFS (HR: 3.01, 95% CI: 1.23–11.30) (66). One specific advantage to the analysis of ctDNA, is the ability to interrogate the molecular pathology of the tumor, without an invasive biopsy, which are rarely done in HCC. In one study of 34 patients in China who underwent liver resection for HCC, and had postoperative ctDNA measured within 90 days of surgery, as well as other protein biomarkers for comparison (AFP, AFP-L3, DCP) to evaluate the potential for identification of MRD. Of the 17 patients that had an early recurrence (<1 year), ctDNA identified 10 (58.8%), approximately double the amount detected by each individual protein marker (67). Another benefit of ctDNA is the combination of powerful sequencing technology such as NGS to detect tumor mutations, which can be monitored longitudinally during the post operative period, or during systemic therapy to monitor response. Indeed, this was performed on 3 patients undergoing TACE and resection, interestingly, in one patient a somatic mutation (HCK p.V174M), which was initially detected after initial TACE treatment, then became undetectable following surgery for initial recurrence, followed by a rapid increase after second recurrence, demonstrating a proof of concept for post operative monitoring (68). Most recently, in a prospective trial of 41 patients with HCC, those with detectable ctDNA preoperatively, were more likely to have an early recurrence than those without, when adjusting for BCLC stage (P<0.05). Additionally, patients with detectable ctDNA at 1- and 4-month time points were more likely to have a shorter time to recurrence (69). Although small, these studies represent an important base from which to build, and the roadmap has already been partially laid out by the implementation of ctDNA in the management of colorectal cancer which has already produced some promising randomized controlled trial data. In a phase II randomized controlled trial of 455 patients undergoing colectomy for stage II colon cancer, patients were randomized to either post operative monitoring with ctDNA [45/291 (15.5%) ctDNA+], versus standard management. Two-year recurrence free survival was not different between ctDNA and standard groups (93.5% and 92.4%, respectively), and importantly, a lower proportion of patients in the ctDNA group received chemotherapy (15% vs. 28%) (70). As more data from implementation in other cancers is produced, it will allow for improved design of clinical trials and application in HCC, that will be more likely to succeed.
Another promising type of liquid biopsy is the detection of CTCs, which has been aided by the recent improvements in NGS, single cell sequencing, and microfluidic technologies (71). The role of CTCs in HCC aims to follow the advancements in applications in colorectal cancer. As in colorectal cancer, CTCs in HCC can be detected in a high percentage of patients, even in those with early-stage disease and in contrast to technologies requiring sequencing, CTC counts are significantly less expensive [$564, ARUP laboratories (12)]. Similar to ctDNA, data regarding sensitivity and specificity of CTC in HCC-specific applications are still emerging, however, in HCC diagnosis, early results are promising with one study reporting a sensitivity and specificity of 75.5% and 86.1%, respectively using a cut-off of 4 CTC/5 mL (72). In one study of 112 patients with HCC undergoing curative resection, CTCs were able to be detected preoperatively in 101 (90.2%) of patients, demonstrating that this method would be widely applicable to a variety of HCC patients. Additionally, they found that patients with a CTC count ≥16 and mesenchymal CTC >2% was a predictor of early recurrence, multifocal intrahepatic recurrence and lung metastasis (73). Similarly, in a prospective study of 42 patients undergoing resection of hepatitis B related HCC, post operative CTC counts of both >2 and >5, as well as increase between pre- and post-operative CTC count, were all associated with decreased progression free survival (P<0.05 for each) (74).
Prediction and monitoring of treatment response
Biomarkers are often utilized in predicting response to immunotherapeutic treatments for a variety of cancers (75). A variety of biomarkers at the soluble, cellular, and genomic levels have been investigated in order to better understand which subsets of patients will best respond to immunotherapy. Examples of these include serum proteins, tumor-specific receptor expression patterns, circulating cells, host genomic factors, and aspects of the tumor microenvironment (76). For HCC, particular biomarkers and genetic characteristics have demonstrated a role during clinical trials in predicting efficacy of immunotherapy (Table 3). Additionally, the presence of microbial signatures in the gut microbiome have also been shown to correlate to responses towards cancer immunotherapy.
Table 3
| Biomarker | Trial name | Intervention/Treatment | Phase | Pertinent findings |
|---|---|---|---|---|
| AFP | GO30140 (NCT02715531) | Atezolizumab + bevacizumab | Ib | AFP decrease ≥75% and increase ≤10% from baseline at 6 weeks used to identify responders and patients with disease control, respectively (77) |
| IMbrave 150 (NCT03434379) | Atezolizumab + bevacizumab | III | AFP decrease ≥75% and increase ≤10% from baseline at 6 weeks used to identify responders and patients with disease control, respectively (78) | |
| Celestial (NCT01908426) | Cabozantinib | III | Greater treatment efficacy for patients with AFP ≥400 ng/mL compared to those under this threshold (79) | |
| REACH (NCT01140347) | Ramucirumab | III | Increased overall survival with second-line treatment of ramucirumab compared to placebo in patients with AFP ≥400 ng/mL (80) | |
| REACH-2 (NCT02435433) | Ramucirumab | III | Increased overall survival with second-line treatment of ramucirumab compared to placebo in patients with AFP ≥400 ng/mL (81) | |
| ctDNA/CTC | SORAMIC (NCT01126645) | Sorafenib + radioembolization or radiofrequency ablation | II | Significant correlation seen between higher circulating free DNA levels and survival (82) |
| PD-L1 | CheckMate 040 (NCT01658878) | Nivolumab + ipilimumab | I/II | No significant difference between response rates in PD-L1 positive (≥1%) and negative patients (<1%) (83) |
| KEYNOTE-224 (NCT02702414) | Pembrolizumab | II | Positive PD-L1 expression associated with improved response (84) | |
| CheckMate 459 (NCT02576509) | Nivolumab vs. sorafenib | III | In PD-L1 positive patients, nivolumab monotherapy was shown to produce a higher response rate compared to sorafenib (85) | |
| TMB/MSI | GO30140 (NCT02715531) | Atezolizumab + bevacizumab | Ib | TMB was not associated with treatment response or progression-free survival (77) |
AFP, alpha-feto protein; ctDNA, circulating tumor DNA; CTC, circulating tumor cells; PD-L1, programmed death ligand-1; TMB, tumor mutational burden; MSI, microsatellite instability.
AFP
In addition to its diagnostic and prognostic applications, AFP has been described to predict responses to immunotherapy for HCC patients. In a multicenter study of HCC patients receiving programmed cell death protein-1 (PD-1) blockade treatment, AFP was identified to be a marker associated with therapy response. Namely, baseline pre-treatment AFP levels less than 400 ng/mL were shown to be associated with significantly longer median progression-free survival (PFS) (P<0.05) and OS (P<0.0001) in patients treated with anti-PD-1 (86). Despite the correlation of advanced disease and elevated levels of AFP, increases in this biomarker have also been associated with greater efficacy of cabozantinib treatment in HCC patients with an AFP ≥400 ng/mL compared to those with AFP under this threshold, although mechanisms driving this association remain unclear and require further study (79). In addition, both the REACH and REACH-2 trials demonstrated increased OS (P<0.05) with second-line treatment of ramucirumab compared to placebo in patients with elevated AFP levels (≥400 ng/mL) following intolerance or cancer progression with sorafenib (80,81). Meanwhile, the initial REACH trial showed no statistically significant difference in OS for patients with AFP <400 ng/mL receiving ramucirumab or placebo (80).
AFP also plays a role in monitoring response to immunotherapies. In a study assessing treatment response to atezolizumab + bevacizumab for unresectable HCC, AFP was demonstrated to be a potential biomarker of predicting OS and PFS. AFP cutoffs of ≥75% decrease and ≤10% increase from baseline at 6 weeks were used to determine responders to treatment and those who had disease control, respectively. For the ≥75% decrease cutoff, the sensitivity was 0.59 and sensitivity was 0.89, whereas the sensitivity was 0.77 and specificity was 0.44 for the ≤10% increase cutoff of disease control (87). Additionally, Hsu et al. showed that ≥20% decline in serum AFP levels within three months of treatment was a predictor for objective response (P=0.042) and PFS (P=0.001) (88). Despite some use in certain populations, AFP, as in other applications, lacks sensitivity and wide applicability, highlighting the need for increased biomarker discovery and validation.
CtDNA and CTC
Liquid biopsy techniques have recently been highlighted as methods to identify ctDNA and CTC in patient blood. Findings from these assays have been suggested as biomarkers for metastasis or recurrence, and efforts have also been made to study ctDNA as a predictor for treatment response (89-92). For advanced non-small cell lung cancer (NSCLC), rapid decreases in plasma ctDNA were associated with a significantly higher response rate in patients treated with first line pembrolizumab based therapy (93). Additionally, in prostate cancer, CTC enumeration has been described as a reliable predictor of prognosis and treatment response (94,95). In HCC, Ikeda et al. have suggested ctDNA as a non-invasive test to identify targetable mutations in tumors for treatment (96). A subset study from the SORAMIC trial also explored the value of using cfDNA and ctDNA in advanced HCC and described its use as a potential biomarker for predicting treatment response (97). In a longitudinal analysis of patients with locally advanced and metastatic HCC, changes in CTC count were shown to be correlated with treatment responses to a variety of systemic therapies (70% sorafenib) and was especially useful for disease monitoring in patients without elevated serum AFP levels (98). Techniques to detect ctDNA and CTC demonstrate promise in various cancer types, but further refinement is required to determine its utility in predicting treatment responses in HCC.
PD-L1
Around 10% of tumor cells demonstrate PD-L1 expression in HCC, which is relatively low compared with a variety of other tumor types, including breast [27%, (99)], pancreatic [19%, (100)], gastric [59%, (101)] (102,103). Although PD-L1 is expressed at a relatively low level, this biomarker has nevertheless been extensively explored in the context of immunotherapy. In the phase III CheckMate 459 trial involving patients with PD-L1 positive advanced HCC, nivolumab monotherapy was shown to produce a higher response rate compared to sorafenib as front-line treatment, although significant improvements in OS were not seen (85). Similarly, findings from the phase II KEYNOTE-224 trial demonstrated that positive PD-L1 expression was associated with improved response rates in patients receiving pembrolizumab monotherapy who had been previously treated with sorafenib (84). Conversely, the phase I/II CheckMate 040 trial reported no statistically significant difference between response rates in PD-L1 positive and negative patients (<1%) (104). Notably all three of these trials used a definition of ≥1% PD-L1 expression for outcome analysis, a very low threshold. Overall, there is not an unequivocal body of evidence towards the utility of PD-L1 as a predictive biomarker for immunotherapeutic treatments in HCC and its true value remains to be determined.
Tumor mutational burden (TMB) and microsatellite instability (MSI)
TMB, the total number of mutations in a tumor genome, has been assessed as another possible biomarker for identifying patients with tumors that may be sensitive to biologic and immunotherapy treatments. Tumors with higher number of mutations have been associated with greater levels of neoantigens that may be targeted in immune responses (105). While there has not been a comprehensive assessment of TMB’s role in predicting tumor sensitivity to immunotherapeutic treatments for HCC, various studies have been conducted across multiple types of cancers that have concluded that high TMB is associated with improved survival and greater rates of treatment response in the context of immune checkpoint inhibitors (106,107). For HCC, an assessment of 755 patients with advanced HCC showed a median TMB of 4 mutations/Mb, with only 6 tumors (0.8%) having high TMB (≥20 mutations/Mb). A further small case series (n=17) by the same group showed that there was no association between TMB and tumor response to immune checkpoint inhibitors (108). Thus, further studies are needed to refine the threshold for high TMB and to assess the predictive value of TMB in stratifying and selecting patients that may benefit from biologic and immunotherapies.
DNA mismatch repair occurs as a safeguard in cases of DNA replication errors, however, deficiencies in this mechanism produce a phenotype of MSI leading to a greater probability of mutations (109). Similar to TMB, higher amounts of mutations and a lack of mismatch repair would increase the levels of neoantigens produced to generate immune responses and vulnerability towards immunotherapies. Many studies have linked MSI-high status to increased sensitivity towards immune checkpoint blockade and tumor response rates in various cancer types (110). In HCC, various studies exploring the frequency of MSI-high status, defined as MSI ≥30%, have demonstrated a relatively low prevalence varying from 0% to 18% of patients (111). Thus, like with high TMB, the low frequency of MSI-high status in HCC patients has stalled the performance of sufficiently powered studies to assess the association between MSI-high and predicting sensitivity to immunotherapy. However, given MSI’s demonstrated value in various other cancers, further studies should be conducted to assess its value as a biomarker for predicting tumor sensitivity to immunotherapeutic treatments.
Gut microbiome
In addition to its various roles in regulating other disease processes, the gut microbiota plays an integral role in managing innate and adaptive immune responses, especially in the context of cancer. Alterations in the composition of the gut microbiome have been associated with resistance towards chemotherapy and immune checkpoint inhibitor treatments, as antibiotic removal of gut microbiota have led to cyclophosphamide resistance in mice models (112,113). In humans, a systematic review and meta-analysis of 12,794 cancer patients with a variety of tumor types found that use of antibiotic therapy prior to immunotherapy, was associated with decreased response rates, as well as decreased progression-free and OS (114). Additionally, the enrichment of particular bacterial species, such as Bifidobacterium pseudolongum, Lactobacillus johnsonii, and Olsenella, have been shown to significantly improve immune checkpoint inhibitors efficacy in mouse models in multiple tumor types (115). In HCC specifically, a study examining strains of bacteria in patients receiving immune checkpoint inhibitor treatment for unresectable HCC, appreciable differences were identified in patients with objective responses to treatment and progressive disease (116). Prevotella 9 was identified more frequently with progressive disease, whereas strains of Lachnoclostridium, Lachnospiraceae, and Veillonella were more prevalent in patients with objective responses. Further analysis demonstrated that a microbial signature of Lachnoclostridium enrichment and Prevotella 9 depletion independently predicted greater OS in these patients. Similarly, in HCC patients treated with anti-PD-1, Zheng et al. showed that Akkermansia muciniphila and Ruminococcaceae spp were enriched in treatment responders, whereas Proteobacteria was the predominant bacterial species found in non-responders (117). Overall, these studies highlight the utility of gut microbiota as a potential target to enhance immunotherapeutic treatment responses and as a biomarker for disease monitoring in HCC patients.
Other biomarkers
It is important to note that biomarkers are not limited to tissue based analysis. Imaging already plays a central role in the current management of HCC, both before and after diagnosis. Therefore, advances in tissue based biomarkers will assuredly be accompanied by ongoing advances in imaging technology, and importantly, by new applications of artificial intelligence (AI) throughout all phases of HCC management. Use of AI allows for the rapid and comprehensive incorporation of information from massive datasets, that creates the potential for integration of multiple sources of information such as patient characteristics, histopathologic data, molecular profiling, and imaging features, to aid in clinical decision-making (118,119). Additionally, the field of radiomics (the extraction of mineable data from medical imaging) has significant potential for the development of an imaging based biomarker. Importantly, AI applications in this field should allow for significant increases in data mining power. Radiomic approaches have already been tested in the retrospective setting in HCC, including one of the largest to date which used a training cohort of 177 patients from which radiomic features were extracted from preoperative CT scans and combined with pre- and post-operative clinical features to predict recurrence. The model was then validated in an external cohort of 118 patients at two other institutions and had a concordance index of ≥0.77, (P<0.05) (120). As we look beyond AFP as the workhorse of prognostication in HCC, it is important to maintain a comprehensive view of existing as well as emerging technologies, to allow for optimal growth and development of biomarkers.
Conclusions
With the convergence of both the development of new active systemic therapies including chemo- and immunotherapy, as well as technologies for interrogating tumor molecular pathology, we may be approaching a new age in the management of HCC, where both surgical, locoregional and systemic therapies can not only be tailored to tumor biology, but sequentially monitored by novel biomarkers. There is a need for well-developed clinical trials to test and validate existing biomarkers such as ctDNA and CTC that have shown such promise in other malignancies.
Acknowledgments
Figure and visual abstract created using Biorender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yuman Fong and David Kooby) for the series “Molecular, Protein, and Cellular Markers for HPB Cancers” published in Hepatobiliary Surgery and Nutrition. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-526/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-526/coif). The series “Molecular, Protein, and Cellular Markers for HPB Cancers” was commissioned by the editorial office without any funding or sponsorship. D.A.D. receives payments from Johnson & Johnson for surgical video annotation work not relevant to current work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- Shiels MS, O'Brien TR. Recent Decline in Hepatocellular Carcinoma Rates in the United States. Gastroenterology 2020;158:1503-1505.e2. [Crossref] [PubMed]
- Ho SY, Liu PH, Hsu CY, et al. Evolution of etiology, presentation, management and prognostic tool in hepatocellular carcinoma. Sci Rep 2020;10:3925. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Zhang G, Ha SA, Kim HK, et al. Combined analysis of AFP and HCCR-1 as an useful serological marker for small hepatocellular carcinoma: a prospective cohort study. Dis Markers 2012;32:265-71. [Crossref] [PubMed]
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006;101:524-32. [Crossref] [PubMed]
- Llovet JM, Pinyol R, Kelley RK, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer 2022;3:386-401. [Crossref] [PubMed]
- Pelizzaro F, Cardin R, Penzo B, et al. Liquid Biopsy in Hepatocellular Carcinoma: Where Are We Now? Cancers (Basel) 2021;13:2274. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- 002253: α-Fetoprotein (AFP), Tumor Marker | Labcorp [Internet]. [cited 2023 Feb 24]. Available online: https://www.labcorp.com/tests/002253/fetoprotein-afp-tumor-marker
- Pan Y, Chen H, Yu J. Biomarkers in Hepatocellular Carcinoma: Current Status and Future Perspectives. Biomedicines 2020;8:576. [Crossref] [PubMed]
- Out-of-Pocket Patient Cost Calculator | ARUP Laboratories [Internet]. [cited 2023 Feb 24]. Available online: https://www.aruplab.com/testing/resources/calculator
- Rojas Á, Sánchez-Torrijos Y, Gil-Gómez A, et al. Performance of different biomarkers for the management of hepatocellular carcinoma. Hepatoma Res 2018;4:31.
- Chalasani NP, Porter K, Bhattacharya A, et al. Validation of a Novel Multitarget Blood Test Shows High Sensitivity to Detect Early Stage Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2022;20:173-182.e7. [Crossref] [PubMed]
- Jasirwan COM, Fahira A, Siregar L, et al. The alpha-fetoprotein serum is still reliable as a biomarker for the surveillance of hepatocellular carcinoma in Indonesia. BMC Gastroenterol 2020;20:215. [Crossref] [PubMed]
- Yao M, Zhao J, Lu F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget 2016;7:3702-8. [Crossref] [PubMed]
- Yen YH, Kee KM, Li WF, et al. Stationary Trend in Elevated Serum Alpha-Fetoprotein Level in Hepatocellular Carcinoma Patients. Cancers (Basel) 2023;15:1222. [Crossref] [PubMed]
- Kobeisy MA, Morsy KH, Galal M, et al. Clinical significance of elevated alpha-foetoprotein (AFP) in patients with chronic hepatitis C without hepatocellular carcinoma in upper EGYPT. Arab J Gastroenterol 2012;13:49-53. [Crossref] [PubMed]
- Murugavel KG, Mathews S, Jayanthi V, et al. Alpha-fetoprotein as a tumor marker in hepatocellular carcinoma: investigations in south Indian subjects with hepatotropic virus and aflatoxin etiologies. Int J Infect Dis 2008;12:e71-6. [Crossref] [PubMed]
- Wong LL, Kim CJ, Kwee SA, et al. Alpha-fetoprotein testing for hepatocellular carcinoma may not be helpful in nonalcoholic steatohepatitis. Open J Gastroenterol 2013;3:49-54.
- Ridder DA, Weinmann A, Schindeldecker M, et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer 2022;150:1053-66. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Bai DS, Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep 2017;7:12870. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128-39. [Crossref] [PubMed]
- Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158-65. [Crossref] [PubMed]
- Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis 2008;28:110-22. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- Toyoda H, Kumada T, Osaki Y, et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol 2006;4:1528-36. [Crossref] [PubMed]
- Fox R, Berhane S, Teng M, et al. Biomarker-based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br J Cancer 2014;110:2090-8. [Crossref] [PubMed]
- Wongjarupong N, Negron-Ocasio GM, Mara KC, et al. BALAD and BALAD-2 predict survival of hepatocellular carcinoma patients: a North American cohort study. HPB (Oxford) 2021;23:762-9. [Crossref] [PubMed]
- Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66:552-9. [Crossref] [PubMed]
- Yao FY, Hameed B, Mehta N, et al. Response to letter to the editors. Liver Transpl 2014;20:1285. [Crossref] [PubMed]
- Toyoda H, Kumada T, Kaneoka Y, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 2008;49:223-32. [Crossref] [PubMed]
- Shim JH, Yoon DL, Han S, et al. Is serum alpha-fetoprotein useful for predicting recurrence and mortality specific to hepatocellular carcinoma after hepatectomy? A test based on propensity scores and competing risks analysis. Ann Surg Oncol 2012;19:3687-96. [Crossref] [PubMed]
- Gupta S, Kutty G, Jagar P, et al. Very high alpha-fetoprotein (AFP): A poor prognostic indicator in hepatocellular carcinoma in the modern era. Ann Oncol 2013;24:iv67.
- Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016;122:3430-46. [Crossref] [PubMed]
- 141300: α-Fetoprotein (AFP) With AFP-L3% | Labcorp [Internet]. [cited 2023 Feb 24]. Available online: https://www.labcorp.com/tests/141300/fetoprotein-afp-with-afp-l3
- Lamerz R. AFP isoforms and their clinical significance (overview). Anticancer Res 1997;17:2927-30.
- Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta 2001;313:15-9. [Crossref] [PubMed]
- Cheng J, Wang W, Zhang Y, et al. Prognostic role of pre-treatment serum AFP-L3% in hepatocellular carcinoma: systematic review and meta-analysis. PLoS One 2014;9:e87011. [Crossref] [PubMed]
- 141325: Des-γ-carboxy Prothrombin (DCP) | Labcorp [Internet]. [cited 2023 Feb 24]. Available online: https://www.labcorp.com/tests/141325/des-carboxy-prothrombin-dcp
- Uehara S, Gotoh K, Handa H, et al. Distribution of the heterogeneity of des-gamma-carboxyprothrombin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2005;20:1545-52. [Crossref] [PubMed]
- Tang W, Miki K, Kokudo N, et al. Des-gamma-carboxy prothrombin in cancer and non-cancer liver tissue of patients with hepatocellular carcinoma. Int J Oncol 2003;22:969-75.
- Yang M, Zhang X, Liu J. Prognostic value of des-γ-carboxy prothrombin in patients with hepatocellular carcinoma treated with transarterial chemotherapy: A systematic review and meta-analysis. PLoS One 2019;14:e0225170. [Crossref] [PubMed]
- Genentech: Press Releases | Wednesday, Jan 18, 2023 [Internet]. [cited 2023 Feb 24]. Available online: https://www.gene.com/media/press-releases/14981/2023-01-18/genentechs-tecentriq-plus-avastin-is-the
- Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 2019;165:721-30. [Crossref] [PubMed]
- Zhong FP, Zhang YJ, Liu Y, et al. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2017;96:e8043. [Crossref] [PubMed]
- Liu H, Hu FJ, Li H, Lan T, Wu H. Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2021;13:1833-46. [Crossref] [PubMed]
- Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol 2019;26:1474-93. [Crossref] [PubMed]
- Kim HI, An J, Kim JY, et al. Postresection Period-Specific Hazard of Recurrence as a Framework for Surveillance Strategy in Patients with Hepatocellular Carcinoma: A Multicenter Outcome Study. Liver Cancer 2021;11:141-51. [Crossref] [PubMed]
- McHugh PP, Gilbert J, Vera S, et al. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56-61. [Crossref] [PubMed]
- Lee JC, Hung HC, Wang YC, et al. Risk Score Model for Microvascular Invasion in Hepatocellular Carcinoma: The Role of Tumor Burden and Alpha-Fetoprotein. Cancers (Basel) 2021;13:4403. [Crossref] [PubMed]
- Jiang H, Wei J, Fu F, et al. Predicting microvascular invasion in hepatocellular carcinoma: A dual-institution study on gadoxetate disodium-enhanced MRI. Liver Int 2022;42:1158-72. [Crossref] [PubMed]
- Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848-54. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Grigioni AD, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol 2010;52:880-8. [Crossref] [PubMed]
- Cancer Screening Cost with the Galleri Test | Galleri® [Internet]. [cited 2023 Feb 24]. Available online: https://www.galleri.com/patient/the-galleri-test/cost
- FoundationOne Liquid CDx | Foundation Medicine [Internet]. [cited 2023 Feb 24]. Available online: https://www.foundationmedicine.com/test/foundationone-liquid-cdx
- Guardant360® - Therapy Planning with Blood (Liquid Biopsy) | TherapySelect [Internet]. [cited 2023 Feb 24]. Available online: https://www.therapyselect.de/en/guardant360
- Jiang Y, Jiang Y, Wang S, et al. Optimal sequencing depth design for whole genome re-sequencing in pigs. BMC Bioinformatics 2019;20:556. [Crossref] [PubMed]
- Tran NH, Kisiel J, Roberts LR. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep 2021;3:100304. [Crossref] [PubMed]
- Zhang Z, Chen P, Xie H, et al. Using circulating tumor DNA as a novel biomarker to screen and diagnose hepatocellular carcinoma: A systematic review and meta-analysis. Cancer Med 2020;9:1349-64. [Crossref] [PubMed]
- Iizuka N, Sakaida I, Moribe T, et al. Elevated levels of circulating cell-free DNA in the blood of patients with hepatitis C virus-associated hepatocellular carcinoma. Anticancer Res 2006;26:4713-9.
- Liu H, Yang H, Chen X. Prognostic Value of Circulating Tumour DNA in Asian Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2022;2022:8019652. [Crossref] [PubMed]
- Cai Z, Chen G, Zeng Y, et al. Comprehensive Liquid Profiling of Circulating Tumor DNA and Protein Biomarkers in Long-Term Follow-Up Patients with Hepatocellular Carcinoma. Clin Cancer Res 2019;25:5284-94. [Crossref] [PubMed]
- Cai ZX, Chen G, Zeng YY, et al. Circulating tumor DNA profiling reveals clonal evolution and real-time disease progression in advanced hepatocellular carcinoma. Int J Cancer 2017;141:977-85. [Crossref] [PubMed]
- Zhu GQ, Liu WR, Tang Z, et al. Serial circulating tumor DNA to predict early recurrence in patients with hepatocellular carcinoma: a prospective study. Mol Oncol 2022;16:549-61. [Crossref] [PubMed]
- Tie J, Cohen JD, Lahouel K, et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N Engl J Med 2022;386:2261-72. [Crossref] [PubMed]
- Zhang Q, Rong Y, Yi K, et al. Circulating tumor cells in hepatocellular carcinoma: single-cell based analysis, preclinical models, and clinical applications. Theranostics 2020;10:12060-71. [Crossref] [PubMed]
- Yan QJ, Wang Y, Zhu FY. Application Value of Combined Detection of Peripheral Blood CTCs and cfDNA in Early Screening of Liver Cancer. Journal of Clinical Transfusion and Laboratory Medicine 2020;22:429-34.
- Qi LN, Xiang BD, Wu FX, et al. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res 2018;78:4731-44. [Crossref] [PubMed]
- Ye X, Li G, Han C, et al. Circulating tumor cells as a potential biomarker for postoperative clinical outcome in HBV-related hepatocellular carcinoma. Cancer Manag Res 2018;10:5639-47. [Crossref] [PubMed]
- Tray N, Weber JS, Adams S. Predictive Biomarkers for Checkpoint Immunotherapy: Current Status and Challenges for Clinical Application. Cancer Immunol Res 2018;6:1122-8. [Crossref] [PubMed]
- Spencer KR, Wang J, Silk AW, et al. Biomarkers for Immunotherapy: Current Developments and Challenges. Am Soc Clin Oncol Educ Book 2016;35:e493-503. [Crossref] [PubMed]
- Lee MS, Ryoo BY, Hsu CH, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 2020;21:808-20. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-96. [Crossref] [PubMed]
- Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164-74. [Crossref] [PubMed]
- Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol 2021;75:600-9. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90. [Crossref] [PubMed]
- Spahn S, Roessler D, Pompilia R, et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers (Basel) 2020;12:3830. [Crossref] [PubMed]
- Zhu AX, Dayyani F, Yen CJ, et al. Alpha-Fetoprotein as a Potential Surrogate Biomarker for Atezolizumab + Bevacizumab Treatment of Hepatocellular Carcinoma. Clin Cancer Res 2022;28:3537-45. [Crossref] [PubMed]
- Hsu WF, Chuang PH, Chen CK, et al. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res 2020;10:4547-60.
- Dominguez DA, Wang XW. Impact of Next-Generation Sequencing on Outcomes in Hepatocellular Carcinoma: How Precise Are We Really? J Hepatocell Carcinoma 2020;7:33-7. [Crossref] [PubMed]
- Martin SP, Wang XW. The evolving landscape of precision medicine in primary liver cancer. Hepat Oncol 2019;6:HEP12. [Crossref] [PubMed]
- Uesato Y, Sasahira N, Ozaka M, et al. Evaluation of circulating tumor DNA as a biomarker in pancreatic cancer with liver metastasis. PLoS One 2020;15:e0235623. [Crossref] [PubMed]
- Radovich M, Jiang G, Hancock BA, et al. Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol 2020;6:1410-5. [Crossref] [PubMed]
- Ricciuti B, Jones G, Severgnini M, et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J Immunother Cancer 2021;9:e001504. [Crossref] [PubMed]
- Ahn JC, Teng PC, Chen PJ, et al. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology 2021;73:422-36. [Crossref] [PubMed]
- Pantel K, Hille C, Scher HI. Circulating Tumor Cells in Prostate Cancer: From Discovery to Clinical Utility. Clin Chem 2019;65:87-99. [Crossref] [PubMed]
- Ikeda S, Tsigelny IF, Skjevik ÅA, et al. Next-Generation Sequencing of Circulating Tumor DNA Reveals Frequent Alterations in Advanced Hepatocellular Carcinoma. Oncologist 2018;23:586-93. [Crossref] [PubMed]
- Alunni-Fabbroni M, Rönsch K, Huber T, et al. Circulating DNA as prognostic biomarker in patients with advanced hepatocellular carcinoma: a translational exploratory study from the SORAMIC trial. J Transl Med 2019;17:328. [Crossref] [PubMed]
- Rau KM, Liu CT, Hsiao YC, et al. Sequential Circulating Tumor Cell Counts in Patients with Locally Advanced or Metastatic Hepatocellular Carcinoma: Monitoring the Treatment Response. J Clin Med 2020;9:188. [Crossref] [PubMed]
- Tsang JY, Au WL, Lo KY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat 2017;162:19-30. [Crossref] [PubMed]
- Birnbaum DJ, Finetti P, Lopresti A, et al. Prognostic value of PDL1 expression in pancreatic cancer. Oncotarget 2016;7:71198-210. [Crossref] [PubMed]
- Liu X, Choi MG, Kim K, et al. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract 2020;216:152881. [Crossref] [PubMed]
- He Y, Lu M, Che J, et al. Biomarkers and Future Perspectives for Hepatocellular Carcinoma Immunotherapy. Front Oncol 2021;11:716844. [Crossref] [PubMed]
- Pinato DJ, Mauri FA, Spina P, et al. Clinical implications of heterogeneity in PD-L1 immunohistochemical detection in hepatocellular carcinoma: the Blueprint-HCC study. Br J Cancer 2019;120:1033-6. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Di Federico A, et al. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front Oncol 2021;11:803133. [Crossref] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6. [Crossref] [PubMed]
- Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377:2500-1. [Crossref] [PubMed]
- Ang C, Klempner SJ, Ali SM, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019;10:4018-25. [Crossref] [PubMed]
- Li K, Luo H, Huang L, et al. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int 2020;20:16. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Goumard C, Desbois-Mouthon C, Wendum D, et al. Low Levels of Microsatellite Instability at Simple Repeated Sequences Commonly Occur in Human Hepatocellular Carcinoma. Cancer Genomics Proteomics 2017;14:329-39. [Crossref] [PubMed]
- Liu Y, Baba Y, Ishimoto T, et al. Gut microbiome in gastrointestinal cancer: a friend or foe? Int J Biol Sci 2022;18:4101-17. [Crossref] [PubMed]
- Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971-6. [Crossref] [PubMed]
- Tsikala-Vafea M, Belani N, Vieira K, et al. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int J Infect Dis 2021;106:142-54. [Crossref] [PubMed]
- Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020;369:1481-9. [Crossref] [PubMed]
- Lee PC, Wu CJ, Hung YW, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer 2022;10:e004779. [Crossref] [PubMed]
- Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer 2019;7:193. [Crossref] [PubMed]
- Nam D, Chapiro J, Paradis V, et al. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Rep 2022;4:100443. [Crossref] [PubMed]
- Martinino A, Aloulou M, Chatterjee S, et al. Artificial Intelligence in the Diagnosis of Hepatocellular Carcinoma: A Systematic Review. J Clin Med 2022;11:6368. [Crossref] [PubMed]
- Ji GW, Zhu FP, Xu Q, et al. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology 2020;294:568-79. [Crossref] [PubMed]


