Laparoscopic liver resection for hepatocellular carcinoma with cirrhosis in a single institution
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and the fifth most prevalent cancer worldwide (1,2). The incidence of HCC is associated with an increase in hepatitis B- or C-related cirrhosis. The various treatment options for HCC include hepatic resection, liver transplantation, chemotherapy, transarterial chemoembolization, and local ablative therapy. Liver transplantation is a potentially curative option, but, due to various limitations, such as donor availability, recipient age, and continued alcohol abuse, often it is limited in application (3). Therefore, liver resection is an alternative option that is widely accepted as a potentially curative treatment for HCC in patients with adequate liver function, due to technical advances and improvements in perioperative patient management. However, most patients with HCC have underlying chronic liver disease, and a hepatic resection in the setting of cirrhosis adds an extra degree of difficulty. Intra-abdominal varicies, impaired coagulation, a firm liver parenchyma and the specter of postoperative hepatic failure are all potential obstacles to hepatic resection in the cirrhotic population. In open liver surgery, an extremely long incision is necessary for mobilization and resection of the liver, because the liver is anatomically surrounded by the rib cage. In cirrhotic patients, these surgical procedures can result in significant blood loss or the development of intractable postoperative ascites, because of the destruction of collateral circulation in the abdominal wall and the ligaments surrounding the liver.
Laparoscopic procedures for hepatic surgery have been slow to develop, due to the inherent risk of massive bleeding associated with liver resection. The First International Consensus Conference on Laparoscopic Liver Surgery convened in Louisville, Kentucky, in 2008 (4), and since then, the number of laparoscopic liver resections (LLRs) has increased steadily worldwide. Moreover, the number of HCC cases in which LLR is applied has increased steeply over the past five years, especially in Asia and Europe (5). LLR is associated with reduced blood loss, decreased overall and liver-specific complications, and shorter postoperative hospital stays. In a statement by the second International Consensus Conference for Laparoscopic Liver Resection (6), minor LLR was confirmed to be a standard surgical practice, but it is still in the assessment phase (IDEAL 3) (7) as it becomes adopted by an increasing proportion of surgeons. However, it is unclear whether this applies to the more complex group of patients suffering from cirrhosis (8). Therefore, the aim of this retrospective study was to compare the feasibility and safety of LLR for HCC between non-liver cirrhosis (NLC) patients and liver cirrhosis (LC) patients at a single high-volume laparoscopy center. In addition, we reviewed several comparative studies of the perioperative outcomes between LLR and open liver resection (OLR) for HCC patients, recently reported from Asia.
Patients and methods
This is a retrospective study, based on the prospective collection of patient data from a computerized database of all preoperative, perioperative, and postoperative information. From the beginning of 2000 to the end of 2013, 245 patients underwent various treatments for HCC in the Department of Surgery at School of Medicine, Iwate Medical University in Iwate, Japan. Patients were evaluated before treatment according to a specific protocol that included chest radiography, ultrasonography of the abdomen, 4-phase contrast computed tomography of the abdomen, and blood examinations. During this period, OLR was performed in 99 HCC patients, and LLR in 118. The diagnosis of HCC was confirmed by histologic examination of the resected specimens from all patients. LC was diagnosed using the New Inuyama classification system, which is well known in Japan: F0, no fibrosis; F1, portal fibrosis widening; F3, bridging fibrosis plus lobular distortion; and F4, LC, which corresponds to the New European classifications as follows: F0, no fibrosis; F1, mild fibrosis; F2, moderate fibrosis; F3, severe fibrosis; F4, cirrhosis (9,10). In this study, LC was defined histologically as F4 according to the New Inuyama classification system.
Indication for liver resection
Our inclusion criteria for LLR were a tumor size of less than 10 cm and the absence of severe adhesions, invasion to major vessels, or a need for vessel reconstruction. Liver resection was defined according to the Brisbane 2000 classifications, using the following definition: hemihepatectomy, sectionectomy (anterior, posterior, and medial), bisegmentectomy (for resection of two segments), segmentectomy (for resection of one segment), left lateral sectionectomy, and wedge resection (11).
The indications and types of liver resections at our institute were not modified by the use of laparoscopy, similar to the principle in open OLR, and were determined by tumor size, location, and hepatic function. Our criteria for patient eligibility for hepatectomy were based on three parameters: (I) the presence or absence of ascites; (II) total serum bilirubin level; and (III) an indocyanine green retention rate at 15 minutes (ICG R15). The HCC patients who underwent LLR were divided into NLC-LLR (n=60) and LC-LLR (n=58) groups, and we compare the short-term outcomes between them. We defined short-term outcomes as surgical results and the events ocurred for postoperative 90-days.
Laparoscopic liver resection (LLR)
For LLR, the patient was placed in the supine position with the primary surgeon on the right side of the patient and the first assistant and scopist positioned on the left. When tumor lesions were located in the right lateral sector, the patient was placed in the semi-left lateral position for right lobe mobilization. Trocars were inserted using the open technique, and a continuous carbon dioxide pneumoperitoneum was induced at a pressure of 10 mmHg. If unexpected bleeding from tiny holes in the hepatic vein were encountered during the parenchymal dissection, we often maintained the pneumoperitoneum pressure at 10−12 mmHg. Intraoperative ultrasonography was often performed to evaluate and determine the tumor location and to assist in liver parenchymal resection.
For pure-laparoscopic minor resection, including left lateral sectionectomy, we extended the indication of the laparoscopic approach according to tumor location. For laparoscopic major resection, we developed a surgical procedure for performing major hepatectomy through a small incision using a hanging maneuver. We have named this procedure laparoscopy-assisted major liver resection, as it is not a hand-assisted LLR. After performing a large number of these advanced surgical techniques in laparoscopy-assisted major hepatectomies, in 2009 we developed a pure laparoscopic major hepatectomy technique for benign diseases and malignant tumors.
The key technical points of LLR are currently as follows:
- An intermittent Pringle maneuver is necessary to minimize hemorrhage during hepatic parenchymal dissection;
- If not performing the Pringle maneuver securely, precoagulation along the transection line with radiofrequency ablation (RFA) or microwave coagulation therapy (MCT) is necessary to minimize hemorrhage during hepatic parenchymal dissection;
- If a deep tumor is not visible during laparoscopy, it may be difficult to measure the distance between the tumor and the resection margin. To secure the surgical margin, the parenchymal dissection should be performed along the tributaries of the main hepatic vein or the tumor-feeding Glissonean pedicle, which form the landmarks for the deep transection line;
- During parenchymal dissection, effective suction is mandatory to confirm the bleeding point immediately and to keep the resection plane dry (Figure 1);
- The resected specimen is placed in a plastic bag and externalized through either a slightly enlarged port site, the small incision site in laparoscopy-assisted liver resection, or the newly created suprapubic incision.

Comparison between NLC-LLR and LC-LLR
The study criteria for comparing the NLC-LLR group to the LC-LLR group were as follows.
Preoperative data
The following variables were recorded for each group: sex, age, underlying liver disease status [hepatitis B surface antigen (HBs-Ag) and anti-hepatitis C virus antibody positivity], serum alpha-fetoprotein (AFP) and des-gammacarboxyl prothrombin (DCP) levels, ICGR 15, extent of liver damage (determined according to the criteria of the Liver Cancer Study Group of Japan), Child-Pugh score, and the Japan Integrated Staging (JIS) score (13).
Intraoperative and surgical results
Pathological tumor size, tumor number, and surgical margins were analyzed. Difficult tumor locations were defined as the postero-superior segments of the liver (segments 1, 7, and 8, and the superior part of segment 4) (14). Postoperative ascites or pleural effusions were defined as conditions requiring the use of diuretics, or thoracentesis, or abdominal paracentesis after the removal of the intraoperative placed drain. Bile leakage was defined as continuous drainage with a bilirubin concentration of 20 mg/dL or 1,500 mg/day, lasting 2 days. Liver failure was defined as hyperbilirubinemia (total serum bilirubin concentration >5 mg/dL for more than 5 days.
Statistical analysis
The statistical analyses in this study were performed with Stata 13 (Stata Corporation, College Station, TX, USA). In analyses and comparisons of preoperative covariates and clinical parameters, student’s t-test or the Wilcoxon rank sum test for continuous variables, and the χ2 test or Fisher’s exact test for categorical variables, were used. All categorical data were expressed as number or frequency (%), and all continuous data were the mean ± standard deviation, or the median (25%, and 75% quartile range). P<0.05 was considered statistically significant.
Results
The patients’ characteristics are shown in Table 1. Preoperative tumor location difficulty and surgical margins were similar (P=0.5764, and P=0.7754, respectively) between the NLC-LLR group and the LC-LLR group, although the tumor size in the LC-LLR group was significantly smaller than in the NLC-LLR group (P<0.001). There was no significant difference in the incidence of blood loss and transfusion requirements between the NLC-LLR group and the LC-LLR group, although wedge resections were mainly performed in the LC-LLR group. There was no significant difference in the complication rate between the two groups. The remarkable finding was that there was a significantly lower incidence of postoperative ascites in the LC-LLR group than in the NLC-LLR group. One patient in the NC group died of heart failure and the other patient died of sepsis subsequently to hemorrhagic shock (Table 2).

Full table
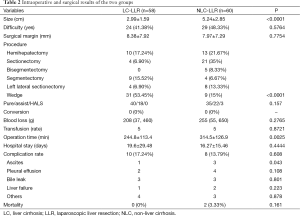
Full table
Discussion
Laparoscopic liver surgery has undergone extreme improvements in recent decades because of important technological developments, advances in preoperative imaging assessments (15), and increasing surgical experience. Due to the development of sophisticated laparoscopic instruments, such as laparoscopic ultrasonic dissectors, sealing energy devices (16), and vascular staplers, laparoscopic liver parenchymal dissection is now considered a feasible and safe alternative to open surgery (17). The number of HCC cases in which LLR is applied has increased steeply over the past five years, especially in Asia, and the rate of conversion to OLR is gradually decreasing. Tables 3,4 show several recent comparative studies between OLR and LLR for HCC patients in Asia (18-27). There were five comparative studies using propensity score matching, although no randomized controlled trials had been published. Propensity score matched analyses have become increasingly used in retrospective cohorts to reduce the impact of selection bias in the comparison of treatments to a non-randomized control group using observational data (28). These comparative studies including a propensity score matched analysis revealed that the most common short-term advantages of LLR were less intraoperative blood loss, shorter hospital stays, and fewer postoperative complications. In comparing the perioperative outcomes of LLR to those of OLR for HCC, using propensity score matching of relatively large data collected from 31 institutions in Japan, Takahara et al. reported that minor LLR in selected patients was a good option as a standard practice for the treatment of HCC.

Full table
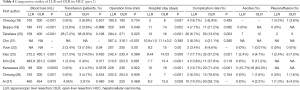
Full table
In HCC patients with cirrhosis, pleural effusion and ascites often develop after conventional OLR. Nevertheless, liver resection remains the main curative procedure. To perform a liver parenchymal transection, a large incision is usually necessary for the mobilization, cutting the surrounding ligaments, and dissection from the diaphragm. Such a large incision and mobilization causes blockage of the collateral circulation around the liver, and consequently results in secondary portal hypertension. With respect to the complications, the frequency of postoperative ascites and pleural effusion after LLR was lower than after OLR. This result might be explained by less destruction of the collateral blood/lymphatic flow by LLR during mobilization of the liver. The reduction of surgery-induced injury with LLR may lower the risk of postoperative liver failure in HCC patients with severe cirrhosis. In the current study, there were no statistically significant differences in the incidence of postoperative morbidity and mortality between the NLC-LLR group and the LC-LLR group. This important finding supported the safety and feasibility of LLR for HCC in patients with cirrhosis.
Initially, cirrhosis was considered a contraindication for LLR. With the growing surgical experiences and the introduction of new equipment, several studies have reported good short-term outcomes after LLR for HCC in patients with cirrhosis. One of the major obstacles of LLR in cirrhotic patients is the risk of massive bleeding, because these patients have a bleeding tendency related to primary hemostasis dysfunction. In the current study, there was no significant difference in the incidence of blood loss and transfusion requirements between the LC-LLR group and the NLC-LLR group, although there was significant difference in the surgical procedure between the two groups. Shehta et al. reported almost the same results as ours (29). This can be explained by the hemostatic effect of pneumoperitoneal pressure, the use of new devices for parenchymal transection, and the use of the Pringle maneuver. Therefore, we performed LLR safety for the superficially localized small tumor, even if the hepatic reserve of the patient was liver damage B or Child-Pugh score B.
In conclusion, according to the Asian experience, it appears that LLR for selected HCC patients with cirrhosis is a feasible and promising procedure that is associated with less blood loss and fewer postoperative complications, especially the incidence of postoperative ascites. Further investigations are clearly warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board.
References
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [PubMed]
- Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis 2009;27:80-92. [PubMed]
- Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: its role: Eastern and Western perspectives. J Hepatobiliary Pancreat Sci 2010;17:443-8. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Hibi T, Cherqui D, Geller DA, et al. International Survey on Technical Aspects of Laparoscopic Liver Resection: a web-based study on the global diffusion of laparoscopic liver surgery prior to the 2nd International Consensus Conference on Laparoscopic Liver Resection in Iwate, Japan. J Hepatobiliary Pancreat Sci 2014;21:737-44. [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. [PubMed]
- Morise Z, Ciria R, Cherqui D, et al. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 2015;22:342-52. [PubMed]
- Sato Y, Iwata T, Yoshimoto J, et al. Clinicalpathological analysis of risk factors for the development of hepatocellular carcinoma after surgery for esophageal varices due to underlying cirrhosis or pre-cirrhosis in the 397 patients. Hepatol Res 2003;25:62-70. [PubMed]
- Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513-20. [PubMed]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351-5. [PubMed]
- Takahara T, Wakabayashi G, Nitta H, et al. Laparoscopic liver resection for hepatocellular carcinoma with cirrhosis in a single institution. Asvide 2015;2:155. Available online: http://www.asvide.com/articles/732
- Ikai I, Takayasu K, Omata M, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol 2006;41:884-92. [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc 2008;22:2344-9. [PubMed]
- Hallet J, Gayet B, Tsung A, et al. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci 2015;22:353-62. [PubMed]
- Scatton O, Brustia R, Belli G, et al. What kind of energy devices should be used for laparoscopic liver resection? Recommendations from a systematic review. J Hepatobiliary Pancreat Sci 2015;22:327-34. [PubMed]
- Otsuka Y, Kaneko H, Cleary SP, et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:363-70. [PubMed]
- Cheung TT, Poon RT, Dai WC, et al. Pure Laparoscopic Versus Open Left Lateral Sectionectomy for Hepatocellular Carcinoma: A Single-Center Experience. World J Surg 2015. [Epub ahead of print]. [PubMed]
- Beppu T, Hayashi H, Okabe H, et al. Hybrid-including endoscopic versus open hepatic resection for patients with hepatocellular carcinoma meeting the Milan criteria: a propensity case-matched analysis. Anticancer Res 2015;35:1583-90. [PubMed]
- Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-7. [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Outcomes of laparoscopic right posterior sectionectomy in patients with hepatocellular carcinoma in the era of laparoscopic surgery. Surgery 2015;158:135-41. [PubMed]
- Yoon SY, Kim KH, Jung DH, et al. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc 2015;29:2628-34. [PubMed]
- Xiao L, Xiang LJ, Li JW, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg Endosc 2015;29:2994-3001. [PubMed]
- Kim H, Suh KS, Lee KW, et al. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc 2014;28:950-60. [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc 2013;27:2592-7. [PubMed]
- Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506-11. [PubMed]
- Ai JH, Li JW, Chen J, et al. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One 2013;8:e72328. [PubMed]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757-63. [PubMed]
- Shehta A, Han HS, Yoon YS, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc 2015. [Epub ahead of print]. [PubMed]


