
Minimally invasive liver resection for huge (≥10 cm) tumors: an international multicenter matched cohort study with regression discontinuity analyses
Introduction
Minimally invasive liver resection (MILR) for liver tumours has been proven to be safe and effective especially for minor liver resections (1-3). The short-term advantages over open surgery include less blood loss, less transfusion requirement, shorter hospital stays and less morbidities (3). The number of laparoscopic and robotic minor liver resections performed worldwide has proliferated in recent years. Nonetheless, most of the major hepatectomies today are still performed via the traditional open approach (1-5). Major liver resection represents an exquisitely complex surgery requiring substantial technical skills and surgeon experience to ensure a safe operation. Hence, many studies recommend that major hepatectomies be carried out in high volume centers. Not surprisingly, the development of major liver resection by the minimally invasive approach has been slow as the learning curve for this approach is long and steep (6-9).
A growing body of literature has demonstrated that MILR can be safely carried out in expert centers, and indications for MILR have been expanding. In Asia, for example, MILR for donor hepatectomy has been adopted cautiously in several high volume centers (10-12). In a similar vein, resection of huge tumours—which were previously considered a contraindication to MILR (13)—was recently reported in a small single-surgeon study, which suggested that if surgeons can overcome the technical difficulties of resecting huge tumours via the minimally-invasive approach, the benefits of minimally invasive surgery could still be maintained (14). Given the paucity of data and potential biases inherent in small single-center studies, a large scale international multicenter study would provide stronger evidence and insight into MILR for huge tumours.
The primary objective of this study was to determine the feasibility and safety of MILR for huge tumours by comparing patients with huge (≥10 cm) tumors against a matched control group of patients with large (3–9.9 cm) tumors. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn-21-327/rc).
Methods
This was a retrospective review of 6,617 patients who underwent RLR and LLR at 21 international centers between 2009–2019. The institutions included performed on average between 20 MILR to over 200 MILR per annum. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All institutions obtained their respective approvals according to their local center’s requirements. This retrospective study on deidentified patient data was approved by the Singapore General Hospital Institution Review Board (2020/2802) and the need for any further board review and patient consent was waived. All anonymized data were collected in the individual centers. These were collated and analyzed centrally at the Singapore General Hospital.
In this study, only patients who underwent pure laparoscopic or robotic-assisted laparoscopic surgery were included. Laparoscopic-assisted (hybrid) and hand-assisted laparoscopic resections were excluded. Notably, there were only 3 cases of hand-assisted laparoscopic resections for huge tumors which were excluded from further analysis.
Other exclusion criteria included patients who underwent donor hepatectomy for transplant, hepatectomy with bilio-enteric anastomoses, resection of cysts and cystic tumors, gallbladder carcinoma and intrahepatic stones.
As this was a multi-center study, the indications and surgical technique for MILR was not standardized. In most cases, the decision to perform MILR for huge tumors was based on an individual surgeon’s comfort level and not any institution protocol. Huge tumors invading major vessels such as the main portal vein or inferior vena cava requiring vascular reconstruction were generally considered an absolute contraindication to MILR (14).
Definitions
Liver resections were defined according to the 2000 Brisbane classification (15). Major resections were classified as resection of 3 or more segments. Additionally, right anterior and right posterior sectionectomies were also considered major resections in this study (16,17). This is due to the wide surface area for parenchymal transection associated with these resections (16,17).
Diameter of the largest lesion was used in the cases of multiple tumors. Huge tumors were defined as tumors with a size ≥10 cm based on histology. Large tumors which were used as the control group were defined as tumors with a size 3–9.9 cm. This size cutoff was used based on the Iwate scoring system which proposed a size cut-off of 3 cm in their difficulty score (18,19).
Difficulty of resections were graded according to the Iwate score (18). Post-operative complications were classified according to the Clavien-Dindo classification and recorded for up to 30 days or during the same hospitalization (20).
Statistical analyses
To enhance the reliability and to assess the robustness of our conclusions to modelling assumptions and various sources of potential bias, we analyzed each comparison using two or more powerful methodologies in the causal inference toolbox.
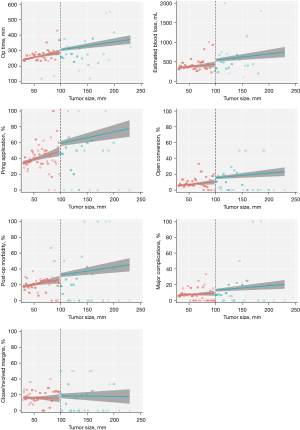
Comparisons between patients who underwent MILR for huge versus large tumors, was performed using 3 statistical frameworks: (I) coarsened-exact matching, (II) nearest neighbor matching based on the Mahalanobis distance, and (III) regression discontinuity analyses. One-to-one coarsened exact matching was used to identify approximately-exact matches between patients with huge or large tumors, taking into account age, ASA status, robotic/laparoscopic approach, prior abdominal or liver surgery, tumor pathology, Child-Pugh score and the presence of cirrhosis and portal hypertension, multifocality, multiple resections, concomitant operations excluding cholecystectomy, major/minor resection (laparoscopic criteria), and difficulty of resection based on the Institute Mutualiste Montsouris (IMM) (21,22) and Iwate grading systems. Coarsening of patient age was done using an automatic binning algorithm based on Sturge’s rule. Next, one-to-two nearest neighbor matching was done based on the Mahalanobis distance metric of baseline covariates shown in Table 1. Finally, we also employed ‘sharp’ regression discontinuity analyses as a further line of sensitivity analysis, as this study design constitutes a powerful quasi-experimental framework (23-26) that is particularly apt when the assignment variable is continuously-measured (as in the case of tumor size) but is dichotomized at a prespecified threshold (e.g., a 10-cm tumor size cutoff in our study). Treatment effects were computed local to the cutoff of 10-cm, with covariance adjustment for the aforementioned baseline characteristics, and the number of bins in regression-discontinuity plots (Figure 1, Figures S1-S15) were chosen based on the evenly-spaced mimicking-variance (ESMV) algorithm.
Table 1
| Characteristics | Entire cohort (n=2,890) | Unmatched cohort | 1:1 coarsened exact matched cohort | 1:2 nearest neighbor matched cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MILR huge (n=205) | MILR large (n=2,685) | P value | MILR huge (n=174) | MILR large (n=174) | P value | MILR huge (n=190) | MILR large (n=380) | P value | ||||
| Median age, MD [IQR], years | 62 [52–70] | 57 [44–70] | 62 [53–70] | <0.001* | 56 [41–69] | 56 [44–66] | 0.988 | 57 [43–70] | 57 [44–68] | 0.811 | ||
| Male sex, n (%) | 1,866/2,886 (64.7) | 108/203 (53.2) | 1,758/2,683 (65.5) | <0.001* | 94/172 (54.7) | 90/174 (51.7) | 0.768 | 103/190 (54.2) | 203/380 (53.4) | 0.852 | ||
| ASA score, n (%) | 0.065 | 1.000 | 0.889 | |||||||||
| I/II | 2,116/2,882 (73.4) | 161/204 (78.9) | 1,955/2,678 (73.0) | 144/174 (82.8) | 144/174 (82.8) | 149/190 (78.4) | 296/380 (77.9) | |||||
| III/IV | 766/2,882 (26.6) | 43/204 (21.1) | 723/2,678 (27.0) | 30/174 (17.2) | 30/174 (17.2) | 41/190 (21.6) | 84/380 (22.1) | |||||
| Robotic, n (%) | 698/2,889 (24.2) | 45/205 (22.0) | 653/2,684 (24.3) | 0.443 | 37/174 (21.3) | 37/174 (21.3) | 1.000 | 42/190 (22.1) | 89/380 (23.4) | 0.724 | ||
| Laparoscopic, n (%) | 2,191/2,889 (75.8) | 160/205 (78.0) | 2,031/2,684 (75.7) | 137/174 (78.7) | 137/174 (78.7) | 148/190 (77.9) | 291/380 (76.6) | |||||
| Previous abdominal surgery, n (%) | 1,031/2,890 (35.7) | 51/205 (24.9) | 980/2,684 (36.5) | 0.001* | 40/174 (23.0) | 40/174 (23.0) | 1.000 | 50/190 (26.3) | 98/380 (25.8) | 0.890 | ||
| Previous liver surgery, n (%) | 160/2,888 (5.5) | 6/205 (2.9) | 154/2,683 (5.7) | 0.090 | 4/174 (2.3) | 4/174 (2.3) | 1.000 | 6/190 (3.2) | 10/380 (2.6) | 0.724 | ||
| Malignant pathology, n (%) | 2,508/2,890 (86.8) | 129/205 (62.9) | 2,379/2,685 (88.6) | <0.001* | 113/174 (64.9) | 113/174 (64.9) | 1.000 | 126/190 (66.3) | 242/380 (63.7) | 0.545 | ||
| Pathology type, n (%) | <0.001* | 1.000 | 0.599 | |||||||||
| HCC/intrahepatic cholangiocarcinoma | 15,92/2,890 (55.1) | 92/205 (44.9) | 1,500/2,685 (55.9) | 82/174 (47.1) | 82/174 (47.1) | 91/190 (47.9) | 165/380 (43.4) | |||||
| CRM and other Mets | 929/2,890 (31.8) | 37/205 (18.0) | 882/2,685 (32.8) | 31/174 (17.8) | 31/174 (17.8) | 35/190 (18.4) | 76/380 (20.0) | |||||
| FNH/adenoma/hemangioma | 379/2,890 (13.1) | 76/205 (37.1) | 303/2,685 (11.3) | 61/174 (35.1) | 61/174 (35.1) | 64/190 (33.7) | 139/380 (36.6) | |||||
| Cirrhosis, n (%) | 738/2,890 (25.5) | 25/205 (12.2) | 713/2,685 (26.6) | <0.001* | 18/174 (10.3) | 18/174 (10.3) | 1.000 | 25/190 (13.2) | 44/380 (11.6) | 0.579 | ||
| Childs Pugh score, n (%) | <0.001* | 1.000 | 0.578 | |||||||||
| No cirrhosis | 2,152/2,890 (74.5) | 180/205 (87.8) | 1,972/2,685 (73.4) | 156/174 (89.7) | 156/174 (89.7) | 165/190 (86.8) | 336/380 (88.4) | |||||
| A | 680/2,890 (23.5) | 20/205 (9.8) | 660/2,685 (24.6) | 15/174 (8.6) | 15/174 (8.6) | 20/190 (10.5) | 29/380 (7.6) | |||||
| B | 58/2,890 (2.0) | 5/205 (2.4) | 53/2,685 (2.0) | 3/174 (1.7) | 3/174 (1.7) | 5/190 (2.6) | 15/380 (3.9) | |||||
| Portal hypertension, n (%) | 192/2,889 (66.5) | 7/204 (3.4) | 185/2,685 (6.9) | 0.056 | 4/174 (2.3) | 4/174 (2.3) | 1.000 | 7/190 (3.7) | 17/380 (4.5) | 0.644 | ||
| Median tumor size, mm, MD [IQR] | 45 [35–62] | 115 [101–135] | 41 [35–57] | <0.001* | 115 [100–134] | 50 [40–65] | <0.001 | 115 [101–134] | 50 [38–70] | <0.001 | ||
| Multiple tumors, n (%) | 614/2,890 (21.2) | 30/205 (14.6) | 584/2,685 (21.8) | 0.016* | 19/174 (10.9) | 19/174 (10.9) | 1.000 | 27/190 (14.2) | 54/380 (14.2) | 1.000 | ||
| Multiple resections, n (%) | 253/2,890 (8.8) | 15/205 (7.3) | 238/2,685 (8.9) | 0.450 | 8/174 (4.6) | 8/174 (4.6) | 1.000 | 13/190 (6.8) | 21/380 (5.5) | 0.536 | ||
| Concomitant operation excluding cholecystectomy, n (%) | 294/2,890 (10.2) | 20/205 (9.8) | 274/2,685 (10.2) | 0.838 | 12/174 (6.9) | 12/174 (6.9) | 1.000 | 19/190 (10.0) | 44/380 (11.6) | 0.568 | ||
| Major/minor resection (minimally-invasive criteria), n (%) | 1,097/2,890 (38.0) | 114/205 (55.6) | 983/2,685 (36.6) | <0.001* | 82/174 (47.1) | 82/174 (47.1) | 1.000 | 104/190 (54.7) | 190/380 (50.0) | 0.289 | ||
| Iwate difficulty score, n (%) | <0.001* | 1.000 | 0.447 | |||||||||
| Low | 195/2,890 (6.7) | 7/205 (3.4) | 188/2,685 (7.0) | 6/174 (3.4) | 6/174 (3.4) | 7/190 (3.7) | 22/380 (5.8) | |||||
| Intermediate | 1,059/2,890 (36.6) | 61/205 (29.8) | 998/2,685 (37.2) | 56/174 (32.2) | 56/174 (32.2) | 57/190 (30.0) | 128/380 (33.7) | |||||
| High | 819/2,890 (28.3) | 52/205 (25.4) | 767/2,685 (28.6) | 46/174 (26.4) | 46/174 (26.4) | 47/190 (24.7) | 93/380 (24.5) | |||||
| Expert | 817/2,890 (28.3) | 85/205 (41.5) | 732/2,685 (27.3) | 66/174 (37.9) | 66/174 (37.9) | 79/190 (41.6) | 137/380 (36.1) | |||||
| IMM resection, n (%) | <0.001* | 0.400 | 0.353 | |||||||||
| Wedge anterior/posterior | 677/2,890 (23.4) | 24/205 (11.7) | 653/2,685 (24.3) | 21/174 (12.1) | 29/174 (16.7) | 23/190 (12.1) | 60/380 (15.8) | |||||
| Left lateral sectionectomy | 487/2,890 (16.9) | 44/205 (21.5) | 443/2,685 (16.5) | 41/174 (23.6) | 33/174 (19.0) | 41/190 (21.6) | 79/380 (20.8) | |||||
| Anterolateral segmentectomy | 391/2,890 (13.5) | 13/205 (6.3) | 378/2,685 (14.1) | 10/174 (5.7) | 10/174 (5.7) | 13/190 (6.8) | 36/380 (9.5) | |||||
| Left hepatectomy | 289/2,890 (10.0) | 37/205 (18.0) | 252/2,685 (9.4) | 25/174 (14.4) | 25/174 (14.4) | 30/190 (15.8) | 64/380 (16.8) | |||||
| Posterolateral segmentectomy | 262/2,890 (9.1) | 11/205 (5.4) | 251/2,685 (9.3) | 10/174 (5.7) | 11/174 (6.3) | 10/190 (5.3) | 19/380 (5.0) | |||||
| Right/extended right hepatectomy | 427/2,890 (14.8) | 56/205 (27.3) | 371/2,685 (13.8) | 50/174 (28.7) | 38/174 (21.8) | 53/190 (27.9) | 87/380 (22.9) | |||||
| Right posterior sectionectomy | 235/2,890 (8.1) | 14/205 (6.8) | 221/2,685 (8.2) | 12/174 (6.9) | 23/174 (13.2) | 14/190 (7.4 | 15/380 (3.9) | |||||
| Central hepatectomy/anterior sectionectomy/extended left hepatectomy | 122/2,890 (4.2) | 6/205 (2.9) | 116/2,685 (4.3) | 5/174 (2.9) | 5/174 (2.9) | 6/190 (3.2) | 20/380 (5.3) | |||||
| IMM difficulty, n (%) | 0.059 | 1.000 | 0.315 | |||||||||
| I | 1,163/2,890 (40.2) | 67/205 (32.7) | 1096/2,685 (40.8) | 62/174 (35.6) | 62/174 (35.6) | 63/190 (33.2) | 139/380 (36.6) | |||||
| II | 681/2,890 (23.6) | 51/205 (24.9) | 630/2,685 (23.5) | 35/174 (20.1) | 35/174 (20.1) | 44/190 (23.2) | 100/380 (26.3) | |||||
| III | 1,046/2,890 (36.2) | 87/205 (42.4) | 959/2,685 (35.7) | 77/174 (44.3) | 77/174 (44.3) | 83/190 (43.7) | 141/380 (37.1) | |||||
*, P value <0.05. MD, mean difference.
Results
Comparison between MILR for huge tumors versus large tumors
There were 2,890 patients who underwent MILR with tumors ≥3 cm which met the study criteria. Of these, 205 patients had huge tumors (≥10 cm) and 2,685 patients had large tumours (≥3 cm). A 1:1 coarsened exact-matching (CEM) and 1:2 Mahalanobis distance-matching (MDM) with consideration of patients’ age, sex, ASA grading, previous abdominal surgery, pathologies, presence of cirrhosis, grading of cirrhosis, presence of portal hypertension, number of tumours, size of tumours, tumour locations, type of operations and the relevant difficulty score was performed. After 1:1 CEM and 1:2 MDM, there were 174 patients in the huge tumor group vs. 174 patients in the large tumor group and 190 huge tumour group vs. 380 patients the large tumour group, respectively.
There was no significant difference between all the demographic data between the 2 groups after both 1:1 CEM and 1:2 MDM. The major indication for surgery was malignancy (66.3% and 63.4% in both groups). Both groups were well-balanced for variables such as American Society of Anesthesiology (ASA) score, presence of liver cirrhosis, posterosuperior locations of tumours as well as proportion of patients undergoing major liver resection.
There was significantly increased intraoperative blood loss, frequency in the application of Pringle maneuver, major morbidity and postoperative stay in the huge tumour group compared to the large tumour group after both 1:1 CEM and 1:2 MDM. Intraoperative blood transfusion rate and open conversion rate were significantly higher in the huge tumor group after 1:2 MDM but not 1:1 CEM. These results are summarized in Tables 1,2.
Table 2
| Outcomes | Entire cohort (n=2,890) | Unmatched cohort | 1:1 coarsened exact matched cohort | 1:2 nearest neighbor matched cohort | Regression discontinuity analysis, β |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MILR huge (n=205) | MILR large (n=2,685) | P value | MILR huge (n=174) | MILR large (n=174) | P value | MILR huge (n=190) | MILR large (n=380) | P value | |||||
| Median operating time, MD [IQR], min | 230 [160–319] | 254 [180–350] | 230 [158–315] | 0.005* | 241 [180–345] | 231 [154–330] | 0.218 | 252 [180–360] | 230 [155–310] | 0.109 | MD: 13.4 mins (−6.4 to 33.3); P=0.185 | ||
| Median blood loss, MD [IQR], mL | 200 [100–450] | 300 [150–500] | 200 [100–450] | <0.001* | 300 [150–500] | 150 [50–400] | 0.001* | 300 [150–500] | 200 [100–412] | <0.001* | MD: 114.6 mls (23.9 to 205.3); P=0.013* | ||
| Blood loss (categories), mL | <0.001* | 0.002* | <0.001* | RD: 16.8 (8.6 to 25.1); P<0.001* | |||||||||
| <300 | 1,517/2,574 (58.9) | 75/173 (43.4) | 1,442/2,401 (60.1) | 64/144 (44.4) | 99/158 (62.7) | 67/160 (41.9) | 212/344 (61.6) | ||||||
| ≥300 | 1,057/2,574 (41.1) | 98/173 (56.6) | 959/2,401 (39.9) | 80/144 (55.6) | 59/158 (37.3) | 93/160 (58.1) | 132/344 (38.4) | ||||||
| Intraoperative blood transfusion, n (%) | 270/2,883 (9.4) | 29/205 (14.1) | 241/2,678 (9.0) | 0.015* | 22/174 (12.6) | 16/174 (9.2) | 0.303 | 28/190 (14.7) | 31/379 (8.2) | 0.021* | RD: 5.1 (0.7 to 9.5); P=0.022* | ||
| Pringle maneuver applied, n (%) | 1,103/2,849 (38.7) | 88/190 (46.3) | 1,015/2,659 (38.2) | 0.026* | 76/161 (47.2) | 60/174 (34.5) | 0.019* | 82/177 (46.3) | 133/376 (35.4) | 0.013* | RD: 9.7 (1.8 to 17.6); P=0.016* | ||
| Median Pringle duration when applied, MD [IQR], min | 30 [20–52] | 34 [19–55] | 30 [20–52] | 0.606 | 36 [20–55] | 30 [17–60] | 0.603 | 34 [20–55] | 30 [20–47] | 0.616 | MD: 2.4 mins (−9.8 to 14.6); P=0.698 | ||
| Open conversion, n (%) | 211/2,890 (7.3) | 24/205 (11.7) | 187 (7.0) | 0.012* | 18/174 (10.3) | 12/174 (6.9) | 0.213 | 24/190 (12.6) | 20/380 (5.3) | 0.004* | RD: 5.3 (1.4 to 9.2); P=0.008* | ||
| Median postoperative stay, MD [IQR], days | 6 [4–8] | 6.9 [4.1–9] | 6 [4–8] | <0.001* | 6.8 [4.1–9.0] | 6.0 [4.1–7] | 0.002* | 6.9 [4.8–9.0] | 6.0 [4.0–7.0] | 0.001* | MD 1.0 days (0.1 to 2.0); P=0.032* | ||
| 30-day readmission, n (%) | 101/2,888 (3.5) | 7/204 (3.4) | 94/2,684 (3.5) | 0.958 | 6/173 (3.5) | 1/174 (0.6) | 0.097 | 7/189 (3.7) | 11/379 (2.9) | 0.631 | RD: 0.7 (−2.1 to 3.5); P=0.615 | ||
| Postoperative morbidity, n (%) | 590/2,890 (20.4) | 51/205 (24.9) | 539/2,685 (20.1) | 0.100 | 42/174 (24.1) | 28/174 (16.1) | 0.063 | 49/190 (25.8) | 78/380 (20.5) | 0.136 | RD: 5.5 (−0.8 to 12.0); P=0.091 | ||
| Major morbidity (Clavien-Dindo grade >2), n (%) | 221/2,889 (7.6) | 23/204 (11.3) | 198/2,685 (7.4) | 0.043* | 19/174 (10.9) | 8/174 (4.6) | 0.026* | 21/189 (11.1) | 27/380 (7.1) | 0.046* | RD: 3.9 (0.1 to 7.7); P=0.043* | ||
| Reoperation, n (%) | 54/2,890 (1.9) | 7/205 (3.4) | 47/2,685 (1.7) | 0.090 | 5/174 (2.9) | 2/174 (1.1) | 0.252 | 6/190 (3.2) | 6/380 (1.6) | 0.120 | RD: 1.7 (−0.9 to 4.4); P=0.208 | ||
| In-hospital mortality, n (%) | 23/2,890 (0.8) | 4/205 (2.0) | 19/2,685 (0.7) | 0.053 | 3/174 (1.7) | 0/174 (0.0) | 0.314 | 4/190 (2.1) | 2/380 (0.5) | 0.109 | RD: 1.1 (−0.7 to 3.0); P=0.223 | ||
| 90-day mortality, n (%) | 39/2,890 (1.3) | 8/205 (3.9) | 31/2,685 (1.2) | 0.001* | 7/174 (4.0) | 2/174 (1.1) | 0.086 | 8/190 (4.2) | 7/380 (1.8) | 0.099 | RD: 2.9 (−0.2 to 6.1); P=0.068 | ||
| Close/ involved margins (≤1 mm) for malignancies, n (%) | 405/2,503 (16.2) | 21/129 (16.3) | 384/2,374 (16.2) | 0.975 | 17/113 (15.0) | 18/113 (15.9) | 0.216 | 20/126 (15.9) | 32/241 (13.3) | 0.515 | RD: 2.8 (−5.9 to 11.6); P=0.523 | ||
‡, regression discontinuity plots are displayed in
Regression discontinuity (RD) analyses generally supported the conclusions of the matched analyses. In RD analyses, we observed that MILR for huge tumors was associated with greater blood loss (MD: 115 mL, 95% CI: 24 to 205 mL; P=0.013) of blood loss, greater need for intraoperative blood transfusion (RD: 5.1%, 95% CI: 0.7% to 9.5%, P=0.022) and Pringle maneuver (RD: 9.7%, 95% CI: 1.8% to 17.6%, P=0.016), open conversion (RD: 5.3%, 95% CI: 1.4% to 9.2%, P=0.008), postoperative stay (MD: 1.0 days, 95% CI: 0.1 to 2.0 days, P=0.032), and major complications (RD: 3.9%, 95% CI: 0.1% to 7.7%, P=0.043) (Table 2). RD plots are presented in Figure 1 and Figures S1-S15.
Discussion
LLR is now an established approach for primary and secondary liver tumors. Since the two international consensus meeting held in Louisville in 2008 and Morioka in 2014, minimally invasive approach to hepatectomy for liver tumours has been safely practiced in many centers with expertise and high volume (27-29). Furthermore, the Asia Pacific consensus statement for minimally invasive hepatectomy for HCC was held in Hong Kong in 2016 (30) and the European guideline meeting on laparoscopic liver surgery was held in Southampton in 2017 (31). These consensus guidelines have further lent credence to the application of minimally invasive technique to treat liver tumors.
Recent studies from high volume centers have demonstrated that major liver resections via the minimally invasive approach demonstrated superior short-term outcome in terms of blood loss, transfusion requirement, complication rate and hospital stay even in patients with liver cirrhosis (8,14,17,32-36). Overall survival appears to be comparable with open approach in patients with liver tumors (32-35). Hence, with these encouraging results, many high-volume centers have been trying to expand the indications of MILR to patient with larger tumours and even huge tumours (14).
The number of huge tumours (≥10 cm) accounted for 10–20% of the total case volume in the current cohort. In this study, the international study group gathered the data from 21 centers with a high volume of MILR and focused on the outcomes of huge liver tumours operated by the minimally invasive approach. Some of the unique technical challenges of performing MILR in patients with huge tumours includes distortion of the normal anatomy, compression of the major portal pedicles, compression of hepatic veins leading to higher venous pressure, limited space for manipulation during surgery, development of large collateral vessels supplying the tumor and presence of tumour invasion or adhesions to adjacent structures. Many of these factors can be anticipated before surgery via careful review of the preoperative cross-sectional imaging.
In this analysis, there was no difference between patients’ pre morbid condition and liver function before the operations after matching. There was significantly and consistently increased intraoperative blood loss, frequency in the application of Pringle maneuver, major morbidity and postoperative stay in the huge tumour group compared to the large tumour group after both 1:1 CEM and 1:2 MDM. These findings were reinforced in RD analyses. Intraoperative blood transfusion rate and open conversion rate were significantly higher in the huge tumour group after only 1:2 MDM but not 1:1 CEM. These reported results were not surprising as similar observations have also been documented with regards to open liver resections for huge tumors (13,37). This is because the presence of a huge tumour limits the exposures of major hepatic veins and worsens liver congestion due to hepatic vein compression (13,37).
During MILR, it has been well-documented that pneumoperitoneum may reduce blood loss especially from the hepatic vein tributaries. In this study, the median blood loss after MILR for huge tumours was only 300 mL which compared favorably to the study by Wakayama et al. which reported a median blood loss of 2,430 mL in a similar cohort of patients undergoing open LR for huge tumours (13). Hence, although in our current study the median blood loss was significantly higher in the huge tumour group compared to the large tumour group, the blood loss was still remarkably low when compared to historic data reporting on open liver resections for huge HCC.
The difference in statistical significance we observed between MDM and CEM with regards to transfusion rate and open conversion rate was not surprising. This is because although CEM better adheres to the “potential outcomes” framework as it identifies “counterfactuals” in the MILR arm whose joint covariate distributions are nearly-identical to their matched counterparts; a major drawback is that the exact matching procedure leads to a smaller matched sample size, which may result in reduced statistical power and increased risk of type 1 and 2 errors (33).
At present, it is important to note that 2 of the most commonly used difficulty scoring systems: the Iwate and IMM system do not take into account huge tumor size (≥10 cm) as an important parameter in determining the difficulty of liver resections (18,19,21,22,38). This is likely because huge tumors were not routinely considered for MILR at the time formulation of these 2 systems. A tumor size cut-off of 3 cm is currently used in the Iwate system, which reflected the rarity of performing MILR for large and huge tumors in most centers. However, since the formulation of both the IMM and the Iwate systems, there has been rapid development of LLR, whereby more complex MILR such as resection of huge tumors has been increasingly performed by surgeons from expert centers. Hence, further updates or a new classification system is needed to provide a more comprehensive coverage of the current situation taking into account that MILR for larger tumors are increasingly performed today and this should be considered an important factor in grading the difficulty of MILR.
The technical challenge of operating in patients with huge tumours can be overcome with different approaches. The Asia Pacific Consensus Statement on Laparoscopic Liver Resection for Hepatocellular Carcinoma held in Hong Kong (30) has highlighted that the use of augmented laparoscopic technology, application of robotic surgery and other adjuncts in hepatectomy can facilitate a surgeon in performing complex MILR. The application of indocyanine green florescence technique in LLR has been widely practiced in recent few years. In LLR, the surgeons had lost his tactile sensation during surgery. The presence of ICG florescence helps to better identify the tumour margin with potential additional advantages of real time illumination of occult lesions and identifying important anatomical landmarks of the liver (30,39). Laparoscopy with high definition and angulation ability may also assist in better identifying lesions and performing resections at a more favorable angle potentially allowing more meticulous dissection of the superiorly located hepatic veins.
It is also important to highlight that hand-assisted MILR was excluded in this analysis. In theory, hand-assistance would be especially useful for huge tumors as the hand would allow more effective manipulation and hence exposure allowing MILR to proceed more quickly with less blood loss (9,40). Nonetheless, to date there is no published data on the use of hand-assistance for huge tumors to support this hypothesis. Furthermore, the use of hand-assistance is usually reported only during the early experience of a center and is utilized as a stepping stone when transitioning from open LR to the totally minimally-invasive approach. In general, most experienced centers today report utilizing hand-assistance sparingly after mounting the learning curve (40). This was reflected in the present analysis whereby only 3 cases of hand-assisted MILR were performed for huge tumors.
The main limitation of the current study is its retrospective nature. Hence, despite matching, residual selection bias may still have confounded the results observed. Furthermore, since this was a multi-center comparative study, there would be differences in the institution or individual surgeon’s MILR experience and surgical techniques deployed during MILR. However, from a different perspective, this could also be viewed as a methodological strength as it reflects current real-world practice and enhances generalizability of our findings as compared to the results of single-center studies. Moreover, a notable strength of this study is the very large sample size of MILR for huge tumors, allowing for a robust statistical analysis.
Conclusions
MILR for huge tumours can be safely performed in expert centers. However, it remains an operation with considerable complexity and high technical requirement, associated with a higher open conversion rate and inferior outcomes when compared to MILR for large tumors. Judicious patient selection is recommended when selecting patients with huge tumors for MILR.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn-21-327/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn-21-327/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn-21-327/coif). Dr. MMV is a consultant for CAVA robotics LLC. Dr. PJ reports a research grant from Intuitive Surgical Deutschland GmbH and personal fees or non-financial support from Johnson & Johnson, Medtronic, AFS Medical, Astellas, CHG Meridian, Chiesi, Falk Foundation, La Fource Group, Merck, Neovii, NOGGO, pharma-consult Peterson, and Promedicis. MS reports personal fees or other support outside of the submitted work from Merck, Bayer, ERBE, Amgen, Johnson & Johnson, Takeda, Olympus, Medtronic, Intuitive. Dr. BKPG has received travel grants and honorarium from Johnson and Johnson and Transmedic the local distributor for the Da Vinci Robot. Dr. JP and Dr. HSH serve as the unpaid editorial board members of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All institutions obtained their respective approvals according to their local center’s requirements. This retrospective study on deidentified patient data was approved by the Singapore General Hospital Institution Review Board (2020/2802) and the need for any further board review and patient consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bryant R, Laurent A, Tayar C, et al. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg 2009;250:103-11. [Crossref] [PubMed]
- Buell JF, Thomas MT, Rudich S, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 2008;248:475-86. [Crossref] [PubMed]
- Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506-11. [Crossref] [PubMed]
- Dagher I, Lainas P, Carloni A, et al. Laparoscopic liver resection for hepatocellular carcinoma. Surg Endosc 2008;22:372-8. [Crossref] [PubMed]
- Goh BKP, Lee LS, Lee SY, et al. Initial experience with robotic hepatectomy in Singapore: analysis of 48 resections in 43 consecutive patients. ANZ J Surg 2019;89:201-5. [Crossref] [PubMed]
- Balasegaram M. Hepatic surgery: a review of a personal series of 95 major resections. Aust N Z J Surg 1972;42:1-10. [Crossref] [PubMed]
- Ban D, Tanabe M, Kumamaru H, et al. Safe Dissemination of Laparoscopic Liver Resection in 27,146 Cases Between 2011 and 2017 From the National Clinical Database of Japan. Ann Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Cheung TT, Ma KW, She WH, et al. Pure laparoscopic versus open major hepatectomy for hepatocellular carcinoma with liver F4 cirrhosis without routine Pringle maneuver - A propensity analysis in a single center. Surg Oncol 2020;35:315-20. [Crossref] [PubMed]
- Dagher I, Gayet B, Tzanis D, et al. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 2014;21:732-6. [Crossref] [PubMed]
- Zhang B, Pan Y, Chen K, et al. Laparoscopy-Assisted versus Open Hepatectomy for Live Liver Donor: Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol 2017;2017:2956749 [Crossref] [PubMed]
- Han HS, Cho JY, Yoon YS, et al. Total laparoscopic living donor right hepatectomy. Surg Endosc 2015;29:184. [Crossref] [PubMed]
- Cherqui D, Ciria R, Kwon CHD, et al. Expert Consensus Guidelines on Minimally Invasive Donor Hepatectomy for Living Donor Liver Transplantation From Innovation to Implementation: A Joint Initiative From the International Laparoscopic Liver Society (ILLS) and the Asian-Pacific Hepato-Pancreato-Biliary Association (A-PHPBA). Ann Surg 2021;273:96-108. [Crossref] [PubMed]
- Wakayama K, Kamiyama T, Yokoo H, et al. Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol 2017;115:324-9. [Crossref] [PubMed]
- Kabir T, Syn NL, Guo Y, et al. Laparoscopic liver resection for huge (≥10 cm) hepatocellular carcinoma: A coarsened exact-matched single-surgeon study. Surg Oncol 2021;37:101569 [Crossref] [PubMed]
- Belghiti J, Clavien PA. The Brisbane 2000 terminology of liver anatomy and resections. HPB (Oxford) 2000;2:333-9. [Crossref]
- Goh BKP, Lee SY, Teo JY, et al. Changing trends and outcomes associated with the adoption of minimally invasive hepatectomy: a contemporary single-institution experience with 400 consecutive resections. Surg Endosc 2018;32:4658-65. [Crossref] [PubMed]
- Goh BKP, Lee SY, Koh YX, et al. Minimally invasive major hepatectomies: a Southeast Asian single institution contemporary experience with its first 120 consecutive cases. ANZ J Surg 2020;90:553-7. [Crossref] [PubMed]
- Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr 2016;5:281-9. [Crossref] [PubMed]
- Ban D, Kudo A, Ito H, et al. The difficulty of laparoscopic liver resection. Updates Surg 2015;67:123-8. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Kawaguchi Y, Tanaka S, Fuks D, et al. Validation and performance of three-level procedure-based classification for laparoscopic liver resection. Surg Endosc 2020;34:2056-66. [Crossref] [PubMed]
- Chen S, Sudharsanan N, Huang F, et al. Impact of community based screening for hypertension on blood pressure after two years: regression discontinuity analysis in a national cohort of older adults in China. BMJ 2019;366:l4064. [Crossref] [PubMed]
- Bor J, Moscoe E, Mutevedzi P, et al. Regression discontinuity designs in epidemiology: causal inference without randomized trials. Epidemiology 2014;25:729-37. [Crossref] [PubMed]
- Bor J, Moscoe E, Bärnighausen T. Three approaches to causal inference in regression discontinuity designs. Epidemiology 2015;26:e28-30; discussion e30. [Crossref] [PubMed]
- Cheung TT, Dai WC, Tsang SH, et al. Pure Laparoscopic Hepatectomy Versus Open Hepatectomy for Hepatocellular Carcinoma in 110 Patients With Liver Cirrhosis: A Propensity Analysis at a Single Center. Ann Surg 2016;264:612-20. [Crossref] [PubMed]
- Goh BKP, Syn N, Lee SY, et al. Impact of liver cirrhosis on the difficulty of minimally-invasive liver resections: a 1:1 coarsened exact-matched controlled study. Surg Endosc 2021;35:5231-8. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Cheung TT, Han HS, She WH, et al. The Asia Pacific Consensus Statement on Laparoscopic Liver Resection for Hepatocellular Carcinoma: A Report from the 7th Asia-Pacific Primary Liver Cancer Expert Meeting Held in Hong Kong. Liver Cancer 2018;7:28-39. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Cheung TT. Laparoscopic liver resection in patients with liver cirrhosis-the path towards standard of care. Hepatobiliary Surg Nutr 2018;7:68-9. [Crossref] [PubMed]
- Goh BKP, Syn N, Koh YX, et al. Comparison between short and long-term outcomes after minimally invasive versus open primary liver resections for hepatocellular carcinoma: A 1:1 matched analysis. J Surg Oncol 2021;124:560-71. [Crossref] [PubMed]
- Nakamura M, Wakabayashi G, Tsuchida A, et al. Precision anatomy for minimally invasive hepatobiliary pancreatic surgery: PAM-HBP Surgery Project. J Hepatobiliary Pancreat Sci 2020; Epub ahead of print. [Crossref] [PubMed]
- Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg 2021;108:196-204. [Crossref] [PubMed]
- Ciria R, Berardi G, Nishino H, et al. A snapshot of the 2020 conception of anatomic liver resections and their applicability on minimally invasive liver surgery. A preparatory survey for the Expert Consensus Meeting on Precision Anatomy for Minimally Invasive HBP Surgery. J Hepatobiliary Pancreat Sci 2021; Epub ahead of print. [Crossref] [PubMed]
- Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (≥10 cm) hepatocellular carcinoma. J Surg Oncol 2016;113:621-7. [Crossref] [PubMed]
- Goh BKP, Prieto M, Syn N, et al. Validation and comparison of the Iwate, IMM, Southampton and Hasegawa difficulty scoring systems for primary laparoscopic hepatectomies. HPB (Oxford) 2021;23:770-6. [Crossref] [PubMed]
- Wang X, Teh CSC, Ishizawa T, et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg 2021;274:97-106. [Crossref] [PubMed]
- Goh BKP, Prieto M, Syn N, et al. Critical appraisal of the learning curve of minimally invasive hepatectomy: experience with the first 200 cases of a Southeast Asian early adopter. ANZ J Surg 2020;90:1092-8. [Crossref] [PubMed]


