
Cholangiocarcinoma: a review of the literature and future directions in therapy
Introduction
Cholangiocarcinoma (CCA) is a group of heterogeneous, rare cancers with an incidence of about 1.26 per 100,000 people (1). The term CCA represents tumors in the intrahepatic or extrahepatic [perihilar (pCCA) vs. distal (dCCA)] bile ducts. Intrahepatic cholangiocarcinoma (iCCA) can often present as an incidental hepatic lesion (2). Saha et al. found an overall growth in the incidence of iCCA in the U.S. over both 40- and 10-year time periods (between 1973 and 2012), with minimal change in the incidence of perihilar and distal CCA (3).
Patients with CCA often present at late stages of the disease with nonspecific symptoms, such as painless jaundice, weight loss, or cholangitis. Prognosis is therefore guarded as these cancers are difficult to diagnose and treat; in fact, approximately half of untreated patients die within 3–4 months due to local tumor progression, bile duct obstruction, liver failure, or sepsis from cholangitis and abscesses. Treatment is palliative in these advanced cases (4) and may be limited to chemotherapy such as gemcitabine and cisplatin vs. upcoming targeted therapies. We present the following article in accordance with the Narrative Review reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-396/rc).
Objectives
The objective of our review of cholangiocarcinoma is to identify important literature over the past 20 years in the form of published research studies, both prospective and retrospective, and highlight the studies we felt were important to the advancement of treatment in cholangiocarcinoma during that time. We focused on surgical studies as well as medical based studies in the realm of palliative treatment.
Methods
We performed a thorough literature search focusing on both retrospective and prospective studies in cholangiocarcinoma. We queried PubMed as well as Google Scholar for our literature search. The literature reviewed primarily consisted of publications from the year 2000 to present published in English. Our discussion included primarily completed studies whose results were published but also included some studies current accruing.
Surgical treatment
Curative intent surgical resection is the standard of care for patients with iCCA (5). Because biliary tract cancers present at an advanced stage, only approximately 20% of tumors are considered resectable (6) compared with 70% for distal biliary duct carcinoma (7).
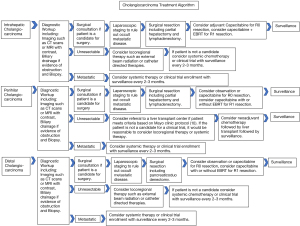
Pre-operative diagnostic workup (Figure 1) (10) in patients with iCCA involves evaluation of radiographic imaging, functional status, and routine blood work. Initial workup identifies a suspicious mass on imaging in the appropriate clinical setting and must be treated as malignancy (8). The typical radiographic characteristics of iCCA on contrast-enhanced imaging studies show a hypodense hepatic lesion without a capsule, and distal biliary dilatation. Rim enhancement can be seen in both arterial and venous phases after administration of the contrast agent (11). Biopsy of the mass to determine diagnosis is necessary prior to surgical resection (8). Radiographic data is a key aspect of surgical planning, as it assesses the potential of a radical R0 tumor resection. Endo et al. demonstrated that multiple hepatic tumors (P<0.001), regional nodal involvement (P=0.012), and large tumor size (P=0.016) independently predicted poor recurrence-free survival (12). A patient’s function status, including ECOG scores, nutritional status, and co-morbidities are also important components of the pre-operative workup (8).
Albumin and total bilirubin levels can be used to predict the risk of post-operative hepatic failure, and pre-operative serum albumin less than 3 g/dL often indicate poor prognosis of iCCA patients (7). Therefore, jaundice-reducing strategies must be pursued in the pre-operative setting. Patients presenting with obstructive jaundice caused by hilar duct invasion should be considered for pre-operative biliary drainage (13). Pre-operative portal vein embolization (PVE) can be performed for patients who are estimated to have a post-operative residual liver deficiency, especially for patients with future liver remnant (FLR) volume less than 30%; this strategy has been observed to promote compensatory proliferation of the reserved residual liver and reduce post-operative complications and mortality (14).
A diagnostic laparoscopy may be considered to rule out unresectable widely metastatic or disseminated disease. The role of a staging laparoscopy in the management of iCCA is not well documented, but may be beneficial in some cases to identify intrahepatic metastases and vascular invasion. Goere et al. demonstrated that 36% of patients with iCCA were found to be unresectable due to laparoscopic detection of peritoneal or intrahepatic metastasis (15). It can serve as an important tool to assess contraindications to surgical resection, including multifocal hepatic disease, lymph nodes metastases beyond the porta hepatis and distant metastatic disease (16). Pre-operative biopsies are not always necessary before proceeding with resection (15). Biopsy should only be pursued once transplant and/or respectability status has been determined. Typically, for patients undergoing resection, a biopsy is not usually necessary. When done, the preferred biopsy approach is an intraluminal biopsy for potential transplant patients (8).
Until 1979, the surgical treatment of iCCA included only bile duct resection, which was associated with numerous debilitating post-operative complications and increased morbidity and mortality. Launois et al. demonstrated the improvement in overall survival (OS) when bile duct resection was combined with hepatic resection (17). Current surgical treatment for iCCA includes R0 resection, lymphadenectomy, total gross resection of the involved biliary tracts, blood vessels and surrounding tissues in adjacent organs, and reconstruction (13). The overall goal of surgery is hepatic resection with negative margins and while major resections are often necessary, wedge and segmental resections are pursued if a negative margin can be achieved (12).
Surgical intervention ideally involves hepatic resection with negative microscopic margins (R0) and a porta hepatis lymphadenectomy (18). Radical resection offers a 5-year survival ranging between 25–45% (19). Most tumors, however, are diagnosed at an advanced stage and the respectability rate is low (6). Compared to patients who undergo R0 resection, R1 resections that result in a microscopically positive margin are associated with a higher risk of recurrence and a shorter overall survival (20). Furthermore, Kim and colleagues demonstrated that adjuvant radiation after surgical resection improves recurrence-free survival in R1 disease due to improvement in loco-regional control (21).
PCCA represents more than 50% of all biliary tract cancers for which radical surgical resection is the only treatment offering a chance of long-term survival (22). Nuzzo et al. conducted an Italian multicenter analysis of 440 patients to evaluate improvements in operative and long-term post-operative results following surgery for pCCA (23). The group found that although surgical techniques have become more aggressive (including more frequent caudate lobectomies, vascular resections, and resections for advanced tumors), median overall survival has increased. In addition, intraoperative blood transfusion rates decreased from 81.0% to 53.2% and mortality decreased from 13.6% to 10.8%; median overall survival significantly increased from 16 to 30 months (P=0.05). For dCCA, pancreaticoduodenectomy procedures are indicated in patients who are considered surgical candidates (21).
Liver transplant (LT)
Although surgical resection is a curative treatment for a number of patients, extensive hilar invasion, bilateral liver involvement, and vascular encasement often preclude curative resection. There are no clearly defined criteria for selecting patients who are candidates for transplant in the setting of cholangiocarcinoma, however based on the Mayo clinic data, patients should have a lesion no larger than 3 cm, without evidence of metastatic disease [based on full body imaging and endoscopic retrograde cholangiopancreatography (ERCP)]. The Mayo protocol also excludes iCCA as well as gall bladder carcinoma (10).
In addition, many patients with underlying primary sclerosing cholangitis (PSC) are unable to successfully undergo surgical resection due to advanced tumor stage (24). LT is efficacious in these patient populations with unresectable iCCA because it avoids the need to achieve tumor-free margins within the liver (25). However, due to highly controversial outcomes, LT is generally not recommended as a routine procedure for iCCA (26).
Currently most patients with iCCA are excluded from transplantation. However, one early report demonstrated that among 28 transplant patients with PSC, 10 patients had small incidental iCCA measuring <1.0 cm in diameter which were found intra-operatively. None of these patients experienced tumor recurrence; the 5-year survival rate was 83%, comparable to that of patients without iCCA. Four patients in this study with known tumors prior liver transplantation had tumor recurrence within 6 months and had a significantly worse outcome (P<0.0001) (27). A Spanish multicenter study reported the outcomes of solitary iCCA <2 cm, referred to as “very early,” in cirrhotic patients and among eight patients with “very early” iCCA, including four incidental tumors, there was a 5-year survival of 73% without tumor recurrence (28). Although further studies are needed to elucidate the selection criteria for LT among patients with iCCA, current literature supports LT in a select patient population with small solitary tumors.
Initial attempts at liver transplantation alone for the treatment of iCCA were faced with high rates of early disease recurrence and poor patient survival (24). The Cincinnati Transplant Registry reported a 28% 5-year survival with a 51% tumor recurrence rate in patients who underwent liver transplantation with no other treatments (29). Spanish liver transplant centers reported similar results with a 30% 3-year survival for their patient cohort that underwent a LT alone (30).
Between 1993 and 2004, Rea and colleagues (31) analyzed combining the benefits of radiotherapy, chemosensitization, and liver transplantation for patients with unresectable iCCA compared to surgical resection. Patient survival was significantly higher after liver transplantation compared to potentially curative resection (P=0.022), with a lower incidence of tumor recurrences in the transplant patients than resection patients, 13% vs. 27%, respectively. Five-year survival after resection in this study was 20%, whereas neoadjuvant therapy in 71 patients followed by subsequent transplantation (in 38 patients) resulted in a five-year survival rate of 82%. This study suggested that LT with neoadjuvant therapy appeared to have a greater efficacy than resection for selected patients with localized, node-negative hilar cholangiocarcinoma. The biggest disadvantage of LT for iCCA is the limitation of donor organ availability (25).
A small single institution experience demonstrated that among twelve patients with iCCA who underwent LT after neoadjuvant chemotherapy in the pre-transplantation setting, there was an overall survival of 100% [95% confidence interval (CI): 100–100%] at 1 year, 83·3% (95% CI: 27.3–97.5%) at 3 years, and 83.3% (95% CI: 27.3–97.5%) at 5 years (32). This study suggested that selecting patients with locally advanced IHCC who show pre-transplant disease stability on neoadjuvant therapy may benefit from liver transplantation. In addition, patients with small solitary tumors or well-differentiated iCCAs could have a more favorable long-term survival after LT (33).
Adjuvant therapy
Several large randomized control trials have been published recently clarifying the role of adjuvant therapy for iCCAs (34), many of these studies are reviewed in our text (Table 1).
Table 1
| Study | Type of study | Subtype | Number of patients eligible for treatment | Treatment | Comparator group | Outcome (resectable after treatment) | Median overall survival | Follow up treatment |
|---|---|---|---|---|---|---|---|---|
| Neoadjuvant therapies | ||||||||
| Le Roy et al. 2017, (35) | Neoadjuvant | iCCA | 74 vs. 82 (surgery alone) | Gemcitabine/Oxaliplatin [44]; Gemcitabine based regimen NOS [4]; Gemcitabine [3]; TACE/RFA [4]; FOLFOX/FOLFIRI [19] | Surgery alone | 53% | OS: 24.1 (Chemotherapy then surgery) vs. 25.7 in surgery group (P=0.391) | Surgery if amenable, chemotherapy if unresectable |
| Zaborowski et al. 2020, (36) | Neoadjuvant | pCCA | 37 | 5-FU + Radiation followed by maintenance capecitabine | None | 70% Response rate (43% pCR) | OS: 83.8 months (pCR), 20.9 months (residual disease) | Surgery |
| Wiedmann et al. 2003, (37) | Neoadjuvant | pCCA | 7 | Photodynamic therapy | None | 83% 1-year disease free survival | N/A | Surgery |
| Rea et al. 2005, (31) | Neoadjuvant | pCCA, iCCA | 71 | 5-FU/Capecitabine+ Radiation followed by Liver Transplant | Resection | |||
| Valle et al. 2009, ABC-01 (38) | Palliative | iCCA, pCCA, dCCA or ampullary carcinoma | 86 | Gemcitabine 1,000 mg/m2 or preceded by cisplatin 25 mg/m2 (on a 2-week in every three-week cycle, 8 cycles) | Gemcitabine 1,000 mg/m2 as a single agent | PFS: 6-month progression-free survival rate from 45.5% (95% CI: 30.5–59.3%) to 57.1% (95% CI: 41.0–70.3%) | Surgery was an option for some patients eligible but was not the end point | |
| Valle et al. 2010, ABC-02 (2) | Palliative | iCCA, pCCA, dCCA or ampullary carcinoma | 410 | Cisplatin (25 mg per square meter of body-surface area) followed by gemcitabine (1,000 mg per square meter), each administered on days 1 and 8, every 3 weeks for eight cycles | Gemcitabine alone (1,000 mg per square meter on days 1, 8, and 15, every 4 weeks for six cycles) for up to 24 weeks | OS: 11.7 months among the 204 patients in the cisplatin–gemcitabine group and 8.1 months among the 206 patients in the gemcitabine group (hazard ratio, 0.64; 95% CI: 0.52–0.80; P<0.001) | ||
| Okusaka et al. 2010 (39) | Palliative | Unresectable CCA | 84 | Cisplatin 25 mg/m2 followed by gemcitabine 1,000 mg/m2 on days 1, 8 of a 21-day cycle (GC-arm) | Single-agent gemcitabine 1,000 mg/m2 on days 1, 8 and 15 of a 28-day cycle (G-arm) | OS: 11.2 months in the cisplatin-gemcitabine group compared with 7.7 months in the gemcitabine-only group (P=0.139) | ||
| Shroff et al. 2019, (40) | Palliative | iCCA, pCCA, dCCA | 60 | Gemcitabine, 1,000 mg/m2, cisplatin, 25 mg/m2, and nab-paclitaxel, 125 mg/m2, on days 1 and 8 of 21-day cycles | OS: 19.2 months, (95% CI: 13.2 months to non-estimable) with 12-month OS rate of 66% (95% CI: 51–78%). Among high-dose recipients, OS was 19.5 months (95% CI: 10.0–NE), with 15 patients having died, and 15.7 months (95% CI: 8.7–NE) among reduced-dose recipients, with 10 patients having died (P=0.39) | Curative surgery | ||
| Adjuvant therapies | ||||||||
| Primrose et al. 2019, BILCAP trial (41) | Adjuvant | iCCA | 447 total (223 received treatment) | Capecitabine 1,250 mg/m2 14 days out of a 21-day cycle for a total of 6 months | Observation | Improved OS in patients with resected cancer when capecitabine used as adjuvant chemotherapy | OS: 51.5 (treatment group) and 36.4 months (observation) (P=0.097) | None |
| Ebata et al. 2018, BCAT (9) | Adjuvant | iCCA | 225 total (117 received treatment) | Gemcitabine 1,000 mg/m2 on days 1, 8, 15 for 28-day cycle for total 6 cycles | Observation | No significant difference in OS between Gemcitabine adjuvant chemotherapy group and observation group | OS: 62.3 vs. 63.8 months in treatment vs. observation group, respectively; hazard ratio 1·01, 95 per cent c.i. 0·70 to 1·45; P=0.964) | None |
| Edeline et al. 2019, PRODIGE (6) | Adjuvant | iCCA | 196 | Gemcitabine 1,000 mg/m2 on day 1 and oxaliplatin 85 mg/m2 on day 2 of a 14-day cycle for 12 cycles | Observation | No benefit of adjuvant GEMOX in resected biliary tract cancers despite adequate tolerance and delivery of the regimen | OS: 75.8 months (treatment arm) vs. 50.8 months in observation arm (P=0.74) | None |
| Stein et al. 2015, ACTICCA-1 (42) | iCCA, pCCA | 781 | Gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 on days 1 and 8 every 3 weeks for 24 weeks | Capecitabine 1,250 mg/m2 on day 1 to 14 every 3 weeks for 24 weeks | Ongoing trial | Ongoing trial | None | |
| Targeted therapies | ||||||||
| Lowery et al. 2019 (43) | Palliative/Targeted | IDH1 + CCA | IDH1 | Ivosedinib 500 mg daily | No dose-limiting toxicities were reported and maximum tolerated dose was not reached | N/A | None | |
| Abou-Alfa et al. 2019 ClarIDHy (44) | Palliative/Targeted | iCCA | 185 (124 received treatment) | IDH1 | Ivosedinib 500 mg daily | PFS was significantly improved with ivosidenib compared with placebo | PFS: 2.7 [95% CI: 1.6–4.2] months vs. 1.4 [1.4–1.6] months; hazard ratio 0.37; 95% CI: 0.25–0.54; one-sided P<0.0001 | None |
| Jayle et al. 2018 (45) | Palliative/Targeted | iCCA | 71 | FGFR2 fusions | Infigratinib 125 mg orally daily for 21 days of 28-day cycles | Clinically meaningful activity after chemotherapy in pts with intrahepatic cholangiocarcinoma containing FGFR2 fusions with ORR (confirmed and unconfirmed) was 31.0% (95% CI: 20.5–43.1%) and the cORR (in pts with potential for confirmation) was 26.9% (95% CI: 16.8–39.1%) | N/A | None |
| Philip et al. 2006 (46) | Palliative/Targeted | Unresectable iCCA | 42 | Epidermal growth factor receptor/human epidermal growth factor receptor 1 | Erlotinib 150 mg daily | Therapeutic benefit for EGFR blockade with erlotinib | PFS: 6 month PFS 17% (95% CI: 7–31%) | None |
| Gruenberger et al. 2010 (47) | Palliative/Targeted | Unresectable CCA | 30 | Epidermal growth factor receptor/human epidermal growth factor receptor 1 | Cetuximab 500 mg/m2 and gemcitabine 1,000 mg/m2 on day 1 with oxaliplatin 100 mg/m2 on day 2, every 2 weeks for 12 cycles | Cetuximab plus GEMOX was well tolerated and had encouraging antitumor activity, leading to secondary resection in a third of patients. Objective response in 19 patients (63%; 95% CI: 56.2–69.8%), of whom three (10%; 3.2–16.8%) achieved complete response, and 16 (53%; 46.2–59.8%) achieved partial response | N/A | Curative surgery, if eligible |
| Malka et al. 2014 (48) | Palliative/Targeted | Unresectable CCA | 150 | Epidermal growth factor receptor/human epidermal growth factor receptor 1 | Gemcitabine 1,000 mg/m2 and oxaliplatin 100 mg/m2 with or without cetuximab 500 mg/m2 repeated every 2 weeks until disease progression or unacceptable toxicity | The addition of cetuximab did not improve OS in EGFR+ Cholangiocarcinoma | PFS: 6.1 months (experimental) vs. 5.5 months (chemo alone). OS: 11.0 months in experimental vs 12.4 months in chemotherapy group | None |
iCCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; dCCA, distal cholangiocarcinoma; pCR, pathological complete response; OS, overall survival; PFS, progression-free survival; IDH1, isocitrate dehydrogenase-1; FGFR, fibroblast growth factor receptor; EGFR, epidermal growth factor receptor; ORR, overall response rate.
In 2019, Primrose et al. were one of the first teams to demonstrate efficacy of adjuvant therapy for resected cholangiocarcinoma or gallbladder cancer. In their phase 3 study, the BILCAP trial, 447 patients with R0 (complete resection with negative margins) disease were randomized to receive capecitabine at a dose of 1,250 mg/m2 14 days out of a 21-day cycle for a total of 6 months compared to observation. Although the intention-to-treat analysis was negative and this study did not meet its primary endpoint of improving overall survival, results demonstrated an improvement in median overall survival (OS) for capecitabine compared to observation alone with median overall survival of 51.5 and 36.4 months, respectively (P=0.097) (41). Prespecified sensitivity and per-protocol analyses suggest that capecitabine can improve overall survival in patients with resected iCCA when used as adjuvant chemotherapy following surgery. Based on these data, capecitabine has become the new standard of care after curative intent resection of biliary tract cancer according to the ASCO guidelines (34).
This publication was soon followed by two gemcitabine-based chemotherapy regimens. As part of the Bile Duct Cancer Adjuvant Trial (BCAT), Ebata et al. (9) published data from Japan in 2018 with 225 patients randomized to gemcitabine at a dose of 1,000 mg/m2 on days 1, 8, and 15 of a 28-day cycle for 6 cycles. The results showed no significant difference in overall survival between the gemcitabine adjuvant chemotherapy group and the observation group (P=0.964).
Next was the PRODIGE-12 trial by Edeline et al. (6) that randomized 196 patients who underwent R0/R1 resection across 33 centers to receive gemcitabine 1,000 mg/m2 on day 1 and oxaliplatin 85 mg/m2 on day 2 of a 14-day cycle for 12 cycles compared to surveillance. Median OS in the chemotherapy group was 75.8 vs. 50.8 months in the observation arm, though this was not statistically significant (P=0.74). While the study was negative, this was a smaller number of patients with lower numbers of node-positive disease and a high proportion of iCCA, which could have underestimated the true benefit of gemcitabine and platinum chemotherapy in the adjuvant setting.
The most current phase III trial is ongoing. ACTICCA-1, adjuvant chemotherapy with Gemcitabine and Cisplatin Compared to the newly established standard regimen of adjuvant Capecitabine is currently in the recruitment stage (42) to clarify the role of standardized adjuvant chemotherapy, particularly in high-risk patients with node positive disease (5). This trial aims to elucidate the superiority of the combination regimen versus the oral monotherapy.
Neoadjuvant therapy
Most patients with iCCA have advanced disease at presentation and relapse despite surgical interventions (18).
Until 2010, there was no specific first-line treatment for metastatic or locally advanced biliary carcinomas. Gemcitabine had been increasingly used to treat hepatobiliary cancer based on its known efficacy in pancreatic cancer (2). Cisplatin is well-known to have a synergistic effect in combination with gemcitabine in other tumors types, including lung (49), bladder (50), and head and neck cancers (51). This is believed to be because of the interactions of these agents within the stroma and effects on tumor-associated macrophages and tumor-associated fibroblasts, augmenting the uptake of chemotherapeutic drugs by tumor cells, leading to apoptosis and depletion of cancerous collagen deposition (52). The Advanced Biliary Cancer (ABC)-01 trial was among the first to show an improvement in 6-month progression-free survival rate from 45.5% (95% CI: 30.5–59.3%) to 57.1% (95% CI: 41.0–70.3%) in a randomized, phase 2 trial comparing gemcitabine alone to gemcitabine in combination with cisplatin in a cohort of 86 patients (38). A similar study design was used in the phase III ABC-02 with a total of 400 patients, which confirmed a significant survival advantage in patients treated with gemcitabine and cisplatin compared to gemcitabine alone for the treatment of advanced biliary cancer (P<0.001) (2).
Prior to these landmark trials, a Japanese trial in 2010 utilized the same treatment regimens as those used in ABC-02 trial and demonstrated a median overall survival of 11.2 months in the cisplatin-gemcitabine group compared with 7.7 months in the gemcitabine-only group (P=0.139) (39). The BINGO trial in 2014 randomly assigned 101 patients to receive gemcitabine plus oxaliplatin with or without cetuximab and found 4-month progression-free survival rates of 54% (95% CI: 43–65%) in the gemcitabine-oxaliplatin only group and 63% (95% CI: 52–74%) in the gemcitabine-oxaliplatin plus cetuximab group (48). To date, the ABC-02 study remains the benchmark for treating newly diagnosed, advanced ICCs.
In 2019, the MD Anderson group of Shroff et al. (40) tested if the addition of nano-albumin bound paclitaxel (nab-paclitaxel) would improve outcomes when added to gemcitabine and cisplatin. Their single-arm, phase II trial enrolled 60 patients and the cohort were compared to historical controls. After an initial concern with hematologic toxicities from higher doses of gemcitabine and nab-pacitaxel, these doses were adjusted and the triplet regimen was found to be safe and well-tolerated. In the experimental group, the median overall survival of the entire cohort was 19.2 months, (95% CI: 13.2 months to non-estimable) with 12-month OS rate of 66% (95% CI: 51–78%). Post hoc analysis showed that median OS among high-dose recipients was 19.5 months (95% CI: 10.0 months to NE), with 15 patients having died, and 15.7 months (95% CI: 8.7 months to NE) among reduced-dose recipients, with 10 patients having died (P=0.39) Median progression-free survival was 11.8 months (95% CI: 6.0 to 15.6 months). In this study, 12 patients out of 60 (20%) were converted from unresectable to resectable disease and subsequently underwent curative surgery; of the twelve, 2 patients achieved a pathologic complete response. These results suggest a synergistic association between the triple combination therapy and patients’ treatment responses and was felt to merit further investigation.
These initial findings have led to the recently opened pivotal phase III study, SWOG 1,815 comparing gemcitabine, cisplatin versus gemcitabine, cisplatin and nab-paclitaxel (53). This study is planned to enroll 268 patients across the United States with a primary endpoint of median overall survival. If positive, this could represent a new standard of care for newly diagnosed, advanced iCCA patients.
The impressive response and conversion rates noted in the phase 2 Shroff study has led to the hypothesis that the same triplet chemotherapy regimen in patients with high-risk, resectable iCCA given in the neoadjuvant setting could improve recurrence free and overall survival. An ongoing multicenter study is thus assessing the feasibility of using gemcitabine, cisplatin and nab-paclitaxel in patients with resectable iCCA. Patients with histologically confirmed iCCA should have: solitary lesions greater than 5 cm, clinically T1b or higher, multifocal disease or satellite lesions within the same lobe but still resectable, presence of major vascular invasions, suspicious regional lymph nodes (N1), absence of distant extrahepatic disease (54). This study could lead to a major breakthrough in neoadjuvant therapy based on a high response rate for patients who have borderline resectable cancer that may not have responded to gemcitabine/cisplatin alone.
The study design calls for four 21-day cycles with the chemotherapy given once per week for 2 weeks with a week off. The doses reflect the triplet dosing that was moved forward for SWOG 1815. Following 4 cycles of treatment, patients will have re-staging scans and surgery if they are surgical candidates. Adjuvant treatment will be allowed per NCCN guidelines and at the discretion of the treating physician. The plan is to enroll 34 patients in the initial phase of the study while monitoring safety data as well as responses. This study will be important in determining the feasibility, safety and efficacy of neoadjuvant therapy in this disease (54).
Targeted therapy
The molecular understanding of iCCA pathogenesis has improved, allowing for new potential molecular biomarkers and novel targeted and immunotherapies (55). The most commonly identified mutations are isocitrate dehydrogenase isoenzyme (IDH1) and KRAS (56). Mutations in IDH1 are present in approximately 15–25% of iCCA tumors, leading to epigenetic and genetic changes promoting oncogenesis via the production of oncometabolite 2-hydroxyglutarate (2-HG) (57). Ivosidenib (AG-120) is an IDH-1 inhibitor that was evaluated in iCCA patients in a phase I trial and 56% of patients treated with this drug had stable disease and 5% had a partial response (43). ClarIDHy, a phase 3 clinical trial which assessed progression-free survival as a primary endpoint in patients receiving AG-120 versus matched placebo found that PFS with AG-120 versus placebo was 2.4 vs. 1.4 months, with 95% CI: 0.25–0.54; P<0.001. There was a trend towards improved median overall survival with ivosidenib (10.8 vs. 9.7 months for placebo; HR 0.69; one-sided P=0.06), with 57% of placebo-treated patients crossing over to ivosidenib (crossover-adjusted median overall survival was 6 months for placebo; HR 0.46; P=0.0008) (44). Based on these data, there may be a role for AG-120 in the neoadjuvant setting in the future.
Fibroblast growth factor receptor 2 (FGFR2) fusions occur in 13–17% of IHCC. In 2018, a phase II trial evaluated infigratinib, an ATP-competitive FGFR1-3-selective oral tyrosine kinase inhibitor, in patients with refractory advanced ICC following chemotherapy with positive FGFR2 fusions. Primary endpoint in this study was confirmed overall response rate (ORR), which was 31% (95% CI: 20.5–43.1%) and in patients with potential for confirmation, confirmed ORR was 26.9% (95% CI: 16.8–39.1%); this study’s findings suggest clinically meaningful activity following chemotherapy in patients with FGFR2 fusion positive IHC (45). The impressive ORR also suggests there may be utility for these targeted agents in the neoadjuvant setting.
Other targeted therapies are aimed at epidermal growth factor receptors (EGFR) and HER2/neu receptor blockers which harbor erbB2 mutations. Erlotinib and cetuximab are EGFR inhibitors and have been tested as second-line monotherapy or in combination with cytotoxic therapy. Philip et al. demonstrated that erlotinib monotherapy resulted in an overall response rate of 10% in patients with advanced iCCA (46). Gruenberger and colleagues further showed that when the EGFR inhibitor cetuximab was combined with gemcitabine oxaliplatin, the overall response rate was 63%, among whom 10% achieved a complete response; one-third of patients who received the combination therapy were able to undergo surgical resection due to dramatic response (47). However, when cetuximab was combined with gemcitabine/cisplatin in a phase II trial, the addition of the EGFR inhibitor did not result in differences in outcomes or disease control (48). Thus, the potential roles for these agents in both the advanced and neoadjuvant settings remain unclear.
In the case of erbB2 mutations, these mutations are significantly less prevalent in patients with iCCA compared to breast and other gastrointestinal malignancies. However, when they are present, they appear to confer a worse prognosis (58). Wiggers et al. also reported a statistically significant higher expression of HER2 in pCCA and dCCA [risk ratio 0.22 (95% CI: 0.07–0.65)] than in iCCA (59). Ongoing trials are testing the efficacy of HER2 targeting agents in pCCA and dCCA. This is also the case in patients with mutations in the AKT/mTOR pathway. Presence of these mutations are associated with a worse prognosis; Goyal et al. conducted a phase II trial in 2017 of an allosteric AKT inhibitor (MK2206) which was prematurely terminated due to no objective clinical activity in patients with cholangiocarcinoma (60).
Immunotherapy
As seen with other gastrointestinal malignancies, mismatch repair (MMR) deficiency is present in only 5% of iCCAs (61). The KEYNOTE-016 and KEYNOTE-158 trials demonstrated that among patients with MMR-deficient tumors, these tumors are sensitive to immune-checkpoint blockade with overall response rates ranging from 37–57% (62). Although MSI-H tumors are rare, anti-PD-1/PD-L1 monoclonal antibodies exert some antitumor activity in a subset of advanced biliary tract cancers (63). Ueno et al. reported the results of KEYNOTE-158 study evaluating antitumor activity and safety of pembrolizumab in 104 patients with advanced biliary tract cancers with prior progression/intolerance on standard therapy. ORR was 5.8% and found that among the 99 patients in whom MSI status was evaluated, none were MSI-H. PD-L1 expression by IHC assay demonstrated that 61 of 95 tumor samples expressed PD-L1 and ORR was 6.6% (95% CI: 1.8–15.9%) and 2.9% (95% CI: 0.1–15.3%) among patients who were PD-L1 positive and negative, respectively (64). Given limited single agent activity, ongoing studies are looking at novel immunotherapy combinations to enhance efficacy. However, only if improved ORRs are noted could these approaches be considered in the neoadjuvant setting.
Conclusions
iCCA, while rare, is a significantly understudied malignancy. The poor overall survival makes this a disease state that needs significant improvement in the treatment algorithm. Studies showing the addition of chemotherapy to current backbones or new backbones are necessary given that recent studies have shown overall survival benefit (40). The advent of next generation sequencing and mutation detection has allowed us to identify potential therapeutic targets and ongoing studies have shown limited success to date. The best data supporting current treatment modalities remains in the transplant space. Currently, most patients present at advanced stage disease, therefore improving detection methods in the future including with circulating tumor DNA or better screening modalities could increase the number of patients found in early stages that may allow us to treat more patients with surgical resection or transplantation. The role of targeted treatment will also improve and the field may see a silver bullet similar to other diseases in the future, but that may be years away. Current treatments have improved survival marginally with targeted therapy. With newer therapies being directed towards CCA specific mutations, we hope to see larger survival benefits and also improved quality of life.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-396/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-396/coif). RTS reports grants from Merck, Exelixis Pharmaceuticals, Halozyme, Pieris, and Taiho; non-financial support from Seattle Genetics, QED, Debiopharm, Agios, Clovis, and Incyte, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cholangiocarcinoma Patel T. Nat Clin Pract Gastroenterol Hepatol 2006;3:33-42. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. The Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189-200. [Crossref] [PubMed]
- Ejaz A, Cloyd JM, Pawlik TM. Advances in the Diagnosis and Treatment of Patients with Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2020;27:552-60. [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009;69:259-70. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Hepatobiliary Cancers (Version 5.2020). Accessed October 20, 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192-202. [Crossref] [PubMed]
- Rosen CB, Heimbach JK, Gores GJ. Surgery for cholangiocarcinoma: the role of liver transplantation. HPB 2008;10:186-9. [Crossref] [PubMed]
- Valls C, Gumà A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdominal Imaging 2000;25:490-6. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic Cholangiocarcinoma. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Wang Q, Gurusamy KS, Lin H, et al. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2008;CD005444. [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers. Surgical Endoscopy 2006;20:721-5. [Crossref] [PubMed]
- de Jong MC, Hong SM, Augustine MM, et al. Hilar cholangiocarcinoma: tumor depth as a predictor of outcome. Arch Surg 2011;146:697-703. [Crossref] [PubMed]
- Launois B, Campion JP, Brissot P, et al. Carcinoma of the Hepatic Hilus Surgical Management and the Case for Resection. Ann Surg 1979;190:151-7. [Crossref] [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic Cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. [Crossref] [PubMed]
- Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48:308-21. [Crossref] [PubMed]
- Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Kim MY, Kim JH, Kim Y, et al. Postoperative radiotherapy appeared to improve the disease free survival rate of patients with extrahepatic bile duct cancer at high risk of loco-regional recurrence. Radiat Oncol J 2016;34:297-304. [Crossref] [PubMed]
- Ramia JM. Hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:113. [Crossref] [PubMed]
- Nuzzo G, Giuliante F, Ardito F, et al. Improvement in Perioperative and Long-term Outcome After Surgical Treatment of Hilar Cholangiocarcinoma. Arch Surg 2012;147:26. [Crossref] [PubMed]
- Rosen CB, Nagorney DM, Wiesner RH, et al. Cholangiocarcinoma Complicating Primary Sclerosing Cholangitis. Ann Surg 1991;213:21-5. [Crossref] [PubMed]
- Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for Cholangiocarcinoma: When and for Whom? Surg Oncol Clin N Am 2009;18:325-37. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Goss JA, Yersiz H, Shackleton CR, et al. In Situ Splitting Of The Cadaveric Liver For Transplantation. Transplantation 1997;64:871-7. [Crossref] [PubMed]
- Sapisochín G. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J Hepatol 2015;7:2396. [Crossref] [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [Crossref] [PubMed]
- Robles R, Figueras J, Turrión V, et al. Liver transplantation for peripheral cholangiocarcinoma: Spanish experience. Transplant Proc 2003;35:1823-4. [Crossref] [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8. [Crossref] [PubMed]
- Lunsford KE, Javle M, Gaber AO, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma - Authors reply. Lancet Gastroenterol Hepatol 2018;3:529-30. [Crossref] [PubMed]
- Facciuto ME, Singh MK, Lubezky N, et al. Tumors With Intrahepatic Bile Duct Differentiation in Cirrhosis. Transplantation 2015;99:151-7. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47. [Crossref] [PubMed]
- Zaborowski A, Heneghan H, Houlihan D, et al. The Irish experience of the Mayo Protocol for unresectable hilar cholangiocarcinoma. HPB 2020;22:S335. [Crossref] [PubMed]
- Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma. Cancer 2003;97:2783-90. [Crossref] [PubMed]
- Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009;101:621-7. [Crossref] [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [Crossref] [PubMed]
- Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol 2019;5:824. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomized, controlled, multicenter, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Lowery MA, Burris HA, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol 2019;4:711-20. [Crossref] [PubMed]
- Abou-Alfa G, Mercade TM, Javle M, et al. ClarIDHy: A global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann Oncol 2019;30:v872-3. [Crossref]
- Javle M, Kelley RK, Roychowdhury S, et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann Oncol 2018;29:mdy424-030. [Crossref]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II Study of Erlotinib in Patients With Advanced Biliary Cancer. J Clin Oncol 2006;24:3069-74. [Crossref] [PubMed]
- Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol 2010;11:1142-8. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Crinò L, Scagliotti G, Marangolo M, et al. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 1997;15:297-303. [Crossref] [PubMed]
- von der Maase H, Sengelov L, Roberts JT, et al. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, With Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J Clin Oncol 2005;23:4602-8. [Crossref] [PubMed]
- Hitt R, Castellano D, Hidalgo M, et al. Phase II trial of cisplatin and gemcitabine in advanced squamous-cell carcinoma of the head and neck. Ann Oncol 1998;9:1347-9. [Crossref] [PubMed]
- Zhang J, Miao L, Guo S, et al. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J Control Release 2014;182:90-6. [Crossref] [PubMed]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000 Feb 29- . Identifier NCT03768414, Gemcitabine Hydrochloride and Cisplatin With or Without Nab-Paclitaxel in Treating Patients With Newly Diagnosed Advanced Biliary Tract Cancers; 2018 Dec 7 [cited 2020 Oct 20]. Available online: https://clinicaltrials.gov/ct2/show/NCT03768414
- Shroff RT. Down-staging locally advanced disease. Hepatobilliary Surg Nutr 2019;8:AB023. [Crossref]
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. [Crossref] [PubMed]
- Zhu AX, Borger DR, Kim Y, et al. Genomic Profiling of Intrahepatic Cholangiocarcinoma: Refining Prognosis and Identifying Therapeutic Targets. Ann Surg Oncol 2014;21:3827-34. [Crossref] [PubMed]
- Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol 2019;10:751-65. [Crossref] [PubMed]
- Sia D, Tovar V, Moeini A, et al. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene 2013;32:4861-70. [Crossref] [PubMed]
- Wiggers JK, Ruys AT, Koerkamp BG, et al. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol 2014;29:1582-94. [Crossref] [PubMed]
- Goyal L, Zheng H, Yurgelun MB, et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017;123:1979-88. [Crossref] [PubMed]
- Silva VWK, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockage in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020;55:15-26. [Crossref] [PubMed]
- Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 2018;29:mdy282-009. [Crossref]


