
Histologic and molecular features of small well-differentiated pancreatic neuroendocrine tumors associated with the development of liver metastases
The incidence of neuroendocrine neoplasms (NEN) has grown by a factor of 6.4 between 1973 and 2012 (1). According to the United States Surveillance, Epidemiology, and End Results (SEER), some 50% of all NEN, 3.56×105, are gastroenteropancreatic (GEP) in origin, including 1.05, 10.4, 0.48×105 of the small intestine, rectum, and pancreas, respectively. Like the Spanish group, others have reported a higher incidence of pancreatic NEN (P-NEN) (2). Given its typically indolent course, GEP-NENs are the second most prevalent tumors of the digestive system, surpassed only by colorectal cancer, underscoring the importance of understanding them, their diagnosis, and efficient treatment strategies.
In 2017, the World Health Organization (WHO) updated its classification which is probably the only universally accepted tool to stratify patients with NEN into prognostic groups, integrating clinical and pathological variables, such as grade, Ki-67 proliferative index, tumor size, TNM stage, lymphovascular invasion, and functionality (3). However, the cut-off values used are somewhat arbitrary and group them into three grades (G) (G1: Ki-67 <3%, G2: Ki-67 3–20%, and G3: Ki-67 >20%), which entails the loss of prognostic information that the Ki-67% would contribute as a continuous variable (2). Moreover, grade and Ki-67 depend on a pathologist, with potential inter-observer variability.
Different studies have demonstrated that, unlike with other digestive cancers, grade or Ki-67 have greater prognostic weight in NENs than stage (4). Thus, the SEER data show a median overall survival (mOS) of >30 years, 10.2 years, and 12 months for localized, regional, and distant NENs, respectively (1). For their part, mOS was 16.2 years, 8.3 years, and 10 months para G1, G2, and G3 NEN, respectively. These data reveal the worse prognosis of G3–4, with a mOS of less than 12 months regardless of stage and location, than advanced NEN. In contrast and unlike G3 NEN, the behavior of well-differentiated neoplasms is deemed fairly unpredictable and impacted by well-known factors such as site, proliferative index, and by others that have yet to be established, such as different genetic factors (5). In the SEER series, the best prognosis was associated with NENs in the rectum (mOS: 24.6 years) and appendix (>30.0 years), while P-NENs (3.6 years) and NENs in the lung (5.5 years) had the worst prognosis (1). Data from 2,813 cases in the Spanish Group of Neuroendocrine and Endocrine Tumor registry (R-GETNE) reveal different results, with better prognosis for P-NENs. Prognosis was good for NENs of the appendix, jejunum-ileum, or duodenum; intermediate for gastric or P-NENs, and poor for colon, rectum, hepatobiliary, or esophageal NENs (2). However, these figures are substantially influenced by grade and stage, which were not evenly distributed among different primary tumor sites (2).
Limitations of other previous studies regarding prognostic factors include highly heterogenous NEN populations, with multiple confounding factors and disparate follow-up times that limit their applicability.
In the study by Pea et al., 87 sporadic, unifocal, localized, well-differentiated, grade 1–2, nonfunctional P-NENs <3 cm (6) were included. It was a multicenter (one hospital in the United States and three in Europe), retrospective study of a small, albeit very homogenous, well-characterized series. All the tumors had been resected and patients followed for 5 years. Of them, 32 displayed hepatic recurrence and 55 were tumor-free 5 years out. Twenty-four from each group that coincided, according to WHO grade and tumor size, were selected for analysis.
Clinical, pathological, and molecular variables were evaluated through uni- and multivariate analyses and are exhibited in Table 1. Despite being morphologically similar P-NENs, biological variability was confirmed with potential prognostic implications.
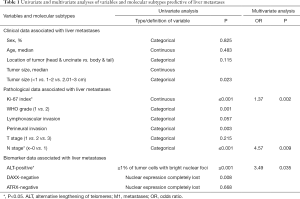
Full table
The three variables associated with developing hepatic metastases were: (I) high Ki-67, assessed as a continuous variable, of between 1–20% (OR 1.369); (II) presence of loco-regional node involvement, N1-stage (OR 4.568), and (III) alternative lengthening of telomeres (ALT)-positivity defined as ≥1% of tumor cells with bright nuclear foci in at least 500 neoplastic cells evaluated (OR 3.486). In this way, the added prognostic value of two well-known clinical-pathological variables, the proliferative index and stage (N), with a third, more recently recognized molecular variable, ALT, was demonstrated (7).
Furthermore, three molecular subtypes were established based on their chromosomal alterations with varying risk of developing hepatic metastases (Table 2). The distribution of P-NENs by molecular subtype was 31%, 40%, and 29% in group 1, 2, and 3, respectively. A correlation was confirmed between recurrent chromosomal gains, ALT, ATRX/DAXX mutation, loss of ATRX/DAXX nuclear protein expression, high proliferative index (73% were G2), and elevated metastatic potential (73% developed hepatic metastases) in group 1. In group 2, 42% developed hepatic metastases despite the fact that most were P-NEN G1; i.e., with low Ki-67. This group exhibited few mutational and chromosomal alterations. Group 3 had the lowest metastatic potential, with 35% hepatic metastases, and was characterized by recurrent chromosome loss, mainly affecting chromosomes 11 (69% MEN1 locus somatic alterations) and 22. No correlation was observed between MEN and ALT. In short, ALT-positive tumors were more aggressive and displayed a higher prevalence of hepatic metastases, whereas those with MEN1 abnormalities were associated with lower risk.

Full table
The authors conclude by suggesting that Ki-67 and ALT be integrated into the diagnostic pathological study, given that they can be useful when deciding on treatment and during follow up.
Given the limited sample size and retrospective nature of the data, independent validations would be appropriate prior to recommending that ALT be extensively used in association with Ki-67 to inform the approach to localized P-NEN; even more so bearing in mind the findings from the multivariate analysis and the study of molecular subgroups.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Nuñez-Valdovinos B, Carmona-Bayonas A, Jiménez-Fonseca P, et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist 2018;23:422-32. [Crossref] [PubMed]
- Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol 2014;25:186-92. [Crossref] [PubMed]
- Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am 2018;47:463-70. [Crossref] [PubMed]
- Martin-Perez E, Capdevila J, Castellano D, et al. Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impact on survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE). Neuroendocrinology. 2013;98:156-68. [Crossref] [PubMed]
- Pea A, Yu J, Marchionni L, et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kim JY, Brosnan-Cashman JA, An S, et al. Alternative Lengthening of Telomeres in Primary Pancreatic Neuroendocrine Tumors Is Associated with Aggressive Clinical Behavior and Poor Survival. Clin Cancer Res 2017;23:1598-606. [Crossref] [PubMed]


