New players in non-alcoholic fatty liver disease induced carcinogenesis: lipid dysregulation impairs liver immune surveillance
Hepatocellular carcinoma (HCC), the primary cancer of the liver, is the fifth most common cancer and the second most common cause of cancer-related death worldwide with an expectancy to increase its incidence over the next 10–20 years (1). Although chronic viral infections [i.e., hepatitis B or C virus (HBV, HCV)], and chronic exposition to hepatotoxic factors such as toxins and alcohol are a major cause of HCC (2), in western countries and especially in the USA, obesity and dysregulated lipid metabolism are emerging as important non-viral factors associated with HCC (3,4). In particular, non-alcoholic fatty liver disease (NAFLD), a manifestation of the metabolic syndrome, is strongly associated with obesity and dyslipidemia (5,6). While most patients with NAFLD remain asymptomatic, about 20–25% progress to develop a more severe chronic hepatic inflammatory disease, defined as non-alcoholic steatohepatitis (NASH) a condition associated with liver fibrosis, cirrhosis and HCC (7,8).
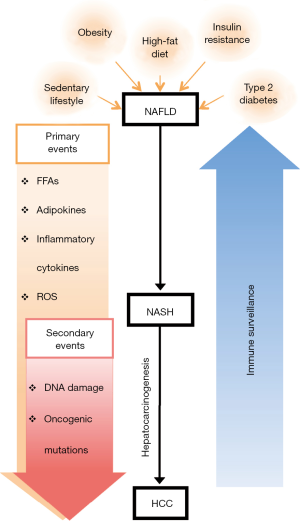
Multiple evidences show that sedentary lifestyle, high-fat diet, obesity and inflammation-associated metabolic disorders such as insulin resistance and type 2 diabetes, can lead to increased intrahepatic secretion of free fatty acids (FFAs), adipokines and pro-inflammatory cytokines and the release of reactive oxygen species (ROS) that induces a chronic low-grade liver inflammatory condition that when unresolved promote hepatocellular death (5,9). In this pathogenic process, repetitive cycles of liver cell death and compensatory hepatocellular proliferation in the presence of mutagenic factors cause direct DNA damage and oncogenic mutations that favor neoplastic transformation and emergence of HCC (10) (Figure 1). Typically this phase of disease can last many years, also because the immune system has the ability to recognize and eliminate pre-tumor or tumor cells before they can cause overt disease in a process defined as tumor immune surveillance or elimination phase of the immune editing theory (11). According to this theory, transformed cells escaping intrinsic anti-tumor pathways are subjected to extrinsic anti-tumor mechanisms that detect and eliminate developing tumors before they become clinically manifest or establish an active equilibrium that control tumor expansion (12). Following these phases, either because of the emergence of tumor cells with reduced immunogenicity or by the engagement of numerous immune suppressor mechanisms involving different immune cell subsets, the anti-tumor immune functions that efficiently controlled nascent tumors, are attenuated thus contributing to HCC emergence and progression (12).
In this context, the study of Ma et al. describes a new functional link between high-fat diet, dysregulation of hepatic lipid metabolism and impaired liver anti-tumor immune surveillance (13). The authors, using different mouse models of NAFLD/NASH and human samples, show that dysregulation of lipid metabolism causes a selective loss of intrahepatic CD4+ but not CD8+ T lymphocytes, leading to accelerated hepatocarcinogenesis (13). In particular, high-fat diet induced intrahepatic accumulation of linoleic acid (C18:2), but not other FFAs, seems to cause primarily the activation of hepatic CD4+ T cells and the selection of α-fetoprotein (AFP)-specific CD4+ T lymphocytes. Following this activation phase, as a consequence of extensive hepatic lipid accumulation, intrahepatic CD4+ T lymphocytes die. The linoleic acid dependent intrahepatic CD4+ T lymphocyte depletion, relies on the high mitochondrial mass and capacity of these cells to produce high levels of ROS, thus causing oxidative cellular damage, finally mediating their selective intrahepatic loss. Thus CD4+ T lymphocytes, after an initial active immune surveillance towards AFP+ tumor cells and probably also to other unknown tumor associated antigens, fail to control tumor progression due to their specific intrahepatic depletion. To further delineate the role of linoleic acid dependent ROS mediated of CD4+ T lymphocyte death, the authors investigated the effect of ROS inhibitors such as catalase or N-acetylcysteine (NAC) on NAFLD-related HCC development. Importantly, catalase and NAC treatment significantly delayed NAFLD-promoted tumor development and this was associated with an effective maintenance of hepatic CD4+ T lymphocytes, suggesting that prevention of intrahepatic CD4+ T lymphocyte death mediates at least partially the anti-tumor effect of catalase and NAC therapy (Figure 2). Consistently with mouse data and with the finding that linoleic acid has also been identified as an important fatty acid in the context of NAFLD in humans, further support the idea that dysregulation of lipid metabolism and selective CD4+ T lymphocyte intrahepatic depletion is part of the pathogenic process of NAFLD/NASH mediated hepatocarcinogenesis. Thus, hepatic CD4+ T lymphocytes and their role in immune surveillance, together with NF-κB dysregulation, innate immune, inflammasome and Toll-like receptor activation represent important players in NAFLD/NASH pathogenesis (13-16), however, leaving open questions. First, the mouse model used does not allow to study lipid derived hepatic carcinogenesis in the context of liver cirrhosis, a conditions highly associated with NASH-mediated HCC development (17). Second, the pathogenic role of activated CD4+ T lymphocytes in the inflammatory phase of NAFLD/NASH can’t be excluded, and pharmacological interventions to restore immune surveillance has to be carefully taken into consideration because of the dual role of immune cells as mediators of liver damage and immune surveillance to tumor cells. Indeed, while metabolic activation of intrahepatic NKT and CD8+ T lymphocytes by lipids contribute to liver damage and promote NASH-driven hepatocarcinogenesis (18), other studies highlight that these same cells may also protect mice from acute-on-chronic liver injury (19), underlining the complex balance between different arms of the immune system. Finally, the definition of the long term consequences of intrahepatic CD4+ T lymphocyte loss in the broad context of microbial immunity to pathogens or microbiota, may be necessary to blow full light into the complex pathogenic mechanism implicated in NAFLD/NASH.
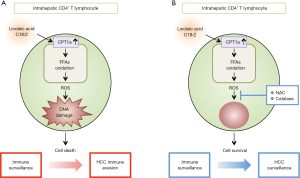
All in all, the results presented by Ma et al., identify obesity-induced selective intrahepatic CD4+ T lymphocyte loss, as crucial new players in the pathogenesis of NAFLD/NASH induced carcinogenesis and suggest that the lack of CD4+ T lymphocyte-mediated immune surveillance plays a critical role in disease progression from NAFLD to HCC representing a new target for specific intervention.
Acknowledgements
Funding: This work was partially supported by grants RF-2011-02346754 and GR-2011-02349580 from the Italian Ministry of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. [Crossref] [PubMed]
- Lee SS, Jeong SH, Byoun YS, et al. Clinical features and outcome of cryptogenic hepatocellular carcinoma compared to those of viral and alcoholic hepatocellular carcinoma. BMC Cancer 2013;13:335. [Crossref] [PubMed]
- Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol 2010;16:3603-15. [Crossref] [PubMed]
- Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology 2004;127:S72-8. [Crossref] [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [Crossref] [PubMed]
- Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565-74. [Crossref] [PubMed]
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012;56:704-13. [Crossref] [PubMed]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013;10:656-65. [Crossref] [PubMed]
- Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J 2010;24:2596-611. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137-46. [Crossref] [PubMed]
- Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253-7. [Crossref] [PubMed]
- Beraza N, Malato Y, Vander Borght S, et al. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut 2008;57:655-63. [Crossref] [PubMed]
- Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179-85. [PubMed]
- Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013;57:577-89. [Crossref] [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [Crossref] [PubMed]
- Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549-64. [Crossref] [PubMed]
- Endig J, Buitrago-Molina LE, Marhenke S, et al. Dual Role of the Adaptive Immune System in Liver Injury and Hepatocellular Carcinoma Development. Cancer Cell 2016;30:308-23. [Crossref] [PubMed]


