
The CRAFITY score can identify patients with hepatocellular carcinoma showing poor response to treatment with atezolizumab and bevacizumab
With great interest we have read the comment of Dr. Khakoo on the recently published CRAFITY score for the prediction of response to anti-programmed death (ligand) 1 [PD-(L)1]-based immunotherapy in patients with unresectable hepatocellular carcinoma (HCC) (1). In his comment, The “CRAFITY” score, as the authors named it, is based on alpha fetoprotein (AFP ≥100 ng/mL) and C-reactive protein (CRP ≥1 mg/dL), and it stratifies patients by likelihood of increased survival and treatment response to subsequent immunotherapy containing systemic treatment (CRAFITY-low, 0 points; CRAFITY-intermediate, 1 point; and CRAFITY-high, 2 points) (2). The CRAFITY score is easy to adopt to clinical practice as the included parameters are available for most patients and measuring them is low cost.
Dr. Khakoo highlighted the potential importance of the CRAFITY score, but also the lack of validation of this score for patients receiving treatment with the current standard of care, the combination treatment with PD-L1 blocker atezolizumab and the vascular endothelial growth factor (VEGF) blocker bevacizumab (atezo/bev). Indeed, the combination treatment with atezo/bev was approved in 2020 by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for systemic treatment-naive patients with unresectable or metastatic HCC based on significantly prolonged overall survival (OS) and progression-free survival (PFS) compared to sorafenib (3,4). Since then, atezo/bev has become the recommended first line systemic treatment for HCC in Europe (5). Prediction of response to this treatment is not established. Although some patients within the patient populations in which the CRAFITY score has been developed and validated include patients treated with atezo/bev (n=25; 25%), their number is limited. To date, there are few real-life cohorts available for such analyses to date owing to the short duration of the approval. However, anticipating an increasing use of this regimen, the performance of the CRAFITY score in this population is of special interest. We have evaluated the performance of the CRAFITY score in a real-world population of HCC patients treated with atezo/bev as a first line systemic treatment.
Of the 45 patients in our analysis [44 Caucasians, 40 males, mean age 68±11 (range, 57–77) years], 40 had liver cirrhosis due to alcohol or fatty liver disease. Patients had intermediate or advanced stage HCC corresponding to Barcelona clinic liver cancer stages B (n=4) or C (n=41). Within the mean observation period of 7.5±5.5 (1.3–21.1) months, 26 patients (58%) showed radiologic disease control (mRECIST criteria), including 15 (33%) with stable disease, 9 (20%) with partial response and 2 (5%) with complete response, whereas 19 (42%) showed progressive disease. Mean OS and mean PFS after initiation of atezo/bev were 10±1.2 [range, 1.3–16.1, 95% confidence interval (CI): 7.6–12.2] and 8.5±1.2 (0.1–12.5, 95% CI: 6.1–11.0) months, respectively. Mean OS was found to be similar among CRAFITY-low [11.4±1.3 (1.5–14.0 months), 95% CI: 8.8–14] or CRAFITY-intermediate patients [10.4±1.6 (1.3–16.1 months), 95% CI: 7.4–13.4; P=n.s.], but significantly shorter in CRAFITY-high patients [6±1.8 (1.3–9.9 months), 95% CI: 2.5–9.5; P=0.018] (Figure 1A). Similarly, mean PFS was found to be similar among CRAFITY-low [9.7±1.6 (0.7–10.6 months), 95% CI: 6.5–14] or CRAFITY- intermediate patients [8.9±1.6 (0.9–12.5 months), 95% CI: 5.8–12; P=n.s.], but significantly shorter in CRAFITY-high patients [3.7±1.5 (0.1–9.7) months, 95% CI: 0.87–6.54; P=0.03] (Figure 1B).
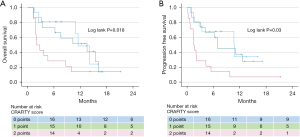
In our patient population treated with atezo/bev, we can confirm the ability of the CRAFITY score to identify patients with poor treatment benefit. Our cohort was retrospective with limited sample size, however, our findings are similar to those of two cohorts of Chinese HCC patients, one treated with the tyrosine kinase inhibitor lenvatinib as monotherapy and the other cohort treated with the combination of lenvatinib plus immunotherapy that were recently published in response to the original publication (6). The accuracy of our observation needs confirmation, as does the question of whether different treatment regimens (e.g., including VEGF inhibitors) require adjusted use of the CRAFITY score. In conclusion, we feel that the CRAFITY score may provide an important impetus for the personalization of systemic HCC treatment and a helpful tool to increase tumour response and patient survival, however, it needs to be separately validated for different treatment regimens.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-280/coif). FvB reported honoraria from Roche, Eisai, Ipsen, MSD, Astra Zeneca and Gilead Sciences; support for attending meetings and/or travel from Gilead Sciences, Eisai; equipment, materials, drugs, medical writing, gifts or other services from Siemens, Diasorin, and Roche, and FvB was on the Data Safety Monitoring Board or Advisory Board of Janssen. TB reported grants or contracts from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Novartis and Sequana Medical; reported consulting fees from Abbvie, Alexion, Bayer, Gilead, Eisai, GSK, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Roche, Sequana Medical, and Shionogi; reported honoraria from Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, and Sequana Medica; reported support for attending meetings and/or travel from Gilead, Abbvie, Intercept, and Janssen. DS reported honoraria from Astellas, BTG, and Novartis; reported support for attending meetings and/or travel from Gilead, Abbvie, Intercept, Janssen, and Johnson & Johnson and DS was on the Data Safety Monitoring Board or Advisory Board of BTG, SIRTEX, and Olympus. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khakoo SI. Immunotherapy for hepatocellular carcinoma: a "CRAFITY" approach to patient stratification. Hepatobiliary Surg Nutr 2022;11:327-9. [Crossref] [PubMed]
- Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol 2022;76:353-63. [Crossref] [PubMed]
- FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. FDA. Updated June 1, 2020. Accessed April 24, 2022. Available online: https://www.bit.ly/3MsBuDd
- Summary of opinion (post-authorisation) EMA/CHMP/488242/2020. EMA CHMP. 2020. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-tecentriq-ii-39_en.pdf
- Vogel A, Martinelli EESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:801-5. [Crossref] [PubMed]
- Yang Y, Ouyang J, Zhou Y, Zhou J, Zhao H. The CRAFITY score: A promising prognostic predictor for patients with hepatocellular carcinoma treated with tyrosine kinase inhibitor and immunotherapy combinations. J Hepatol 2022;77:574-6. [Crossref] [PubMed]


