
Construction of an intrahepatic portosystemic shunt using the magnetic compression technique: preliminary experiments in a canine model
Portal hypertension caused by liver cirrhosis due to hepatitis is a common clinical condition that can cause bleeding from esophageal and gastric varices in severe cases. In 1969, Rösch et al. first described a successful transjugular intrahepatic portosystemic shunt (TIPS) in animal experiments (1). In 1989, Richter et al. first used this technology in clinical practice (2). Clinical practitioners now commonly use TIPS because it is associated with reduced trauma, it significantly reduces portal blood pressure, and it provides a reliable hemostatic effect (3-5). However, a limitation of TIPS is that it has a high rate of stenosis over the long-term, and it can even lead to shunt occlusion. Some studies reported stenosis rates of 17% to 50% at 6 months and 23% to 87% at 12 months (6,7). In recent years, the use of new covered stents has reduced the rate of shunt stenosis after TIPS, but the 12-month stenosis rate is still reported to be 6.9% (8).
The magnetic compression technique (MCT) is a recently developed method that promotes anastomosis by using magnetic forces to establish anastomotic channels in hollow organs. The MCT is therefore a third mode of anastomosis, after manual suture anastomosis and staple anastomosis. More recent studies have described use of the MCT for gastrointestinal anastomosis (9), vascular anastomosis (10), rectovaginal fistula repair (11), and for preparation of therapeutic fistulas (12). In our previous studies, we successfully established side-to-side anastomosis of the portal vein and inferior vena cava in an interventional operation and an open operation using the MCT (13,14). However, no studies have yet examined the use of this technique to establish an intrahepatic portosystemic shunt. We therefore conducted a preliminary study using beagles as an animal model to assess the possibility of constructing an intrahepatic portosystemic shunt using the MCT (Figure 1).
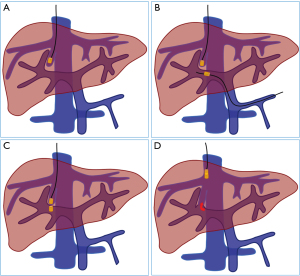
This study was approved by the Committee for the Ethics of Animal Experiments at Xi’an Jiaotong University. Ten beagles (5 males and 5 females) that were 1–6 years old and weighing 12 to 15 kg were obtained from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). Because this was an exploratory study, all animals were included in the experimental investigations, and there was no control group. The magnetic compression device includes two parts: a parent magnet and a daughter magnet. Each magnet is cylindrical, with a diameter of 6 mm and a height of 9 mm, and has a circular hole in the center, with a diameter of 1.5 mm. These magnets were all processed by N45 sintered neodymium iron boron.
The beagles were weighed and then anesthetized using an intravenous injection of pentobarbital sodium (30 mg/kg). With the animal in a supine position, the neck and upper abdomen were shaved and then draped with sterile towels. The right jugular vein (about 20 mm) was dissected and isolated. Then, a median incision (about 15 cm) was made in the upper abdomen; and the splenic vein near the portal vein was about 20 mm long. Five minutes before vascular intervention, a heparin sodium solution (1 mg/kg) was injected through the right great saphenous vein to prevent thrombosis. The right jugular vein and splenic vein were then occluded at the distal end.
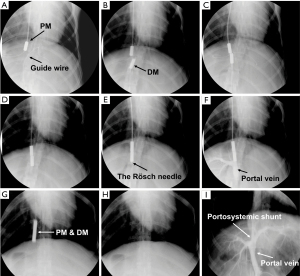
The right jugular vein was incised, a super-smooth guide wire (0.035 inches; TERUMO, Japan) was inserted, and the guide wire was then introduced into the right hepatic vein using X-ray monitoring. The splenic vein was incised, and the guide wire was inserted into the left branch of the portal vein. The parent magnet was inserted, the angiography catheter was fixed on the parent magnet through the super-smooth guide wire, and the parent magnet was then placed into the beginning of the right hepatic vein along the guide wire. The central hole of the daughter magnet was passed through the guide wire into the splenic vein, and a 5-F contrast catheter (Cook, Bloomington, IN, USA) was then used to push the daughter magnet to the beginning of the portal vein bifurcation. At this time, the parent and daughter magnets attracted each other, and the guide wires in the right jugular vein and splenic vein were withdrawn. A Rösch needle was inserted through the catheter of the parent magnet and its tip passed through the parent and daughter magnets into the portal venous system. The contrast medium was then pushed through the catheter of the parent magnet to determine that the daughter magnet and the parent magnet were located in the portal vein and hepatic vein, respectively. An indwelling catheter was placed in the splenic vein for subsequent angiography, and the abdomen was closed layer by layer.
Each dog was fed in a single cage after surgery. After recovery from anesthesia, there were no restrictions on food and water intake. During the first 3 days after surgery, intramuscular pethidine (1 mg/kg) was injected every 12 h. Subcutaneous heparin (4,100 U) was given for 3 consecutive days beginning on the day after surgery. On the first postoperative day, oral warfarin (1.25 mg/day) was initiated. At 14 days after the operation, intravenous anesthesia was administered. Then, using X-ray monitoring, the catheter in the right neck was pulled, and the contrast catheter, together with the parent and daughter magnets, were removed through the right jugular vein. An angiography was performed immediately through the catheter in the splenic vein to observe the patency of the shunts of the portal and hepatic veins.
We successfully established magnetic compression intrahepatic portosystemic shunts in all 10 beagles. During the operation, the parent magnet was inserted into the right hepatic vein via the right jugular vein, and the daughter magnet was inserted into the left branch of the portal vein via the splenic vein. The entire surgical procedure went smoothly in all 10 beagles. There was no intraoperative bleeding, no postoperative complications such as thrombosis, and the postoperative survival rate was 100%. The mean operation time was 56.80±10.23 min (range, 40–75 min). Two weeks after the operation (7 beagles) and three weeks after the operation (3 beagles), the parent and daughter magnets were successfully extracted, and splenic venography indicated successful establishment of the portosystemic shunt (Figure 2). In this study, we successfully developed a new mode of anastomosis by establishing a vascular shunt between the portal vein and the hepatic venous system using the MCT in ten beagles. The success rate was 100% and the beagles had no intraoperative or postoperative complications, confirming the feasibility of this technique for establishing an intrahepatic portosystemic shunt.
At present, the MCT is mainly used to establish digestive tract anastomoses. In this case, a specially designed magnetic anastomosis ring was used instead of suture anastomosis or staple anastomosis. Our previous magnamosis research on portal hypertension examined the use of different devices to establish a vascular shunt between the portal vein and the inferior vena cava (13), as well as a splenorenal shunt (15), in an effort to reduce portal pressure. During the MCT, the nearby parent and daughter magnets generate an attractive magnetic force. Because of the continuous action of the magnetic force, the portal vein wall and liver parenchyma-hepatic vein wall became ischemic and necrotic, and a new blood flow channel formed.
The spleen of the beagle is not suitable for puncture. We therefore used the daughter magnet for splenic venous incision under laparotomy, and then inserted this magnet into the portal venous system to simulate an interventional operation. Although this is a deficiency in the experimental design, it does not affect the core issue of assessing the MCT for establishment of a portosystemic shunt channel. This preliminary study has several limitations. First, we did not measure portal pressure before and after the MCT surgery, and therefore did not directly confirm the effect of this technique in reducing portal pressure. Second, we performed the MCT in healthy animals, not in animals with portal hypertension.
TIPS have been in clinic for more than 30 years. It has been proved to be a less-invasive and safe technique, and has played an important role in the treatment of portal hypertension. The purpose of this study is to investigate the feasibility of this new technique. Long-term observation and appropriate animal models of cirrhosis are required to determine whether the new technique can reduce the stenosis rate of the shunt. Unfortunately, even with the animal model of cirrhotic dogs, it is difficult to compare the stenosis and obstruction of the shunt established by these two methods through animal experiments, which is the deficiency of this study. The major importance of this study is that it described a new method for establishing vascular bypass using an interventional operation. The shunt established by this new technique has no foreign matter retention and may realize the endothelialization of the shunt, which may improve the long-term patency. However, we cannot yet predict whether use of the MCT to establish an intrahepatic portosystemic shunt in humans will be safe or effective. Nonetheless, the MCT appears to have potential for the treatment of certain diseases in vascular surgery. We hope to stimulate some new ideas by introducing this interesting new method to our peers.
Acknowledgments
Funding: This work was supported by the Key Research & Development Program-Social Development of Shaanxi Province of China (2021SF-163) and the Innovation Capability Support Plan of Shaanxi Province of China (2020KJXX-022).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology 1969;92:1112-4. [Crossref] [PubMed]
- Richter GM, Palmaz JC, Nöldge G, et al. The transjugular intrahepatic portosystemic stent-shunt. A new nonsurgical percutaneous method. Radiologe 1989;29:406-11. [PubMed]
- Boyer TD, Henderson JM, Heerey AM, et al. Cost of preventing variceal rebleeding with transjugular intrahepatic portal systemic shunt and distal splenorenal shunt. J Hepatol 2008;48:407-14. [Crossref] [PubMed]
- Boyer TD, Haskal ZJAmerican Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005;41:386-400. [Crossref] [PubMed]
- Boyer TD, Haskal ZJAmerican Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology 2010;51:306. [Crossref] [PubMed]
- Rössle M, Siegerstetter V, Huber M, et al. The first decade of the transjugular intrahepatic portosystemic shunt (TIPS): state of the art. Liver 1998;18:73-89. [Crossref] [PubMed]
- Saxon RS, Ross PL, Mendel-Hartvig J, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology 1998;207:683-93. [Crossref] [PubMed]
- Wang L, Xiao Z, Yue Z, et al. Efficacy of covered and bare stent in TIPS for cirrhotic portal hypertension: A single-center randomized trial. Sci Rep 2016;6:21011. [Crossref] [PubMed]
- Ye D, Zhang MM, Shi AH, et al. Construction of esophagogastric anastomosis in rabbits with magnetic compression technique. J Gastrointest Surg 2021;25:3033-9. [Crossref] [PubMed]
- Wang SP, Yan XP, Xue F, et al. Fast magnetic reconstruction of the portal vein with allogeneic blood vessels in canines. Hepatobiliary Pancreat Dis Int 2015;14:293-9. [Crossref] [PubMed]
- Yan XP, Zou YL, She ZF, et al. Magnet compression technique: a novel method for rectovaginal fistula repair. Int J Colorectal Dis 2016;31:937-8. [Crossref] [PubMed]
- Uygun I, Okur MH, Cimen H, et al. Magnetic compression gastrostomy in the rat. Pediatr Surg Int 2012;28:529-32. [Crossref] [PubMed]
- Yan X, Fan C, Ma J, et al. Portacaval shunt established in six dogs using magnetic compression technique. PLoS One 2013;8:e76873. [Crossref] [PubMed]
- Wang HH, Ma J, Wang SP, et al. Magnetic Anastomosis Rings to Create Portacaval Shunt in a Canine Model of Portal Hypertension. J Gastrointest Surg 2019;23:2184-92. [Crossref] [PubMed]
- Xue F, Li J, Lu J, et al. Splenorenal shunt via magnetic compression technique: a feasibility study in canine and cadaver. Minim Invasive Ther Allied Technol 2016;25:329-36. [Crossref] [PubMed]


