
Multi-disciplinary clinic models for the management of non-alcoholic fatty liver disease
In recent years, there has been an increasing interest in the implementation of multi-disciplinary clinic (MDC) models in the medical field, in an effort to improve coordination of care due to the increasing complexity of medical practice. It is therefore unsurprising that there is such interest in non-alcoholic fatty liver disease (NAFLD), which is now the most prevalent liver disease worldwide.
NAFLD is the hepatic manifestation of the metabolic syndrome and is considered a multifactorial clinical-histopathologic entity, with clinical manifestations that involve a wide range of medical disciplines, so that different specialists are often involved in the management of the same patient. NAFLD is commonly associated with obesity, diabetes mellitus, dyslipidemia, cardiovascular diseases, chronic kidney disease and atherosclerosis, with such conditions being either contributing causes or consequences of NAFLD in a bi-directional mode. It is revealing that the main cause of death in unselected patients with NAFLD is cardiovascular disease, with liver-related complications only third in frequency.
Existing evidence points to an association of liver fibrosis with cardiovascular disease, with important implications for the management of such patients. Vilar-Gomez and co-authors reported on the pivotal role of fibrosis in patients with advanced NAFLD in a 10-year follow-up study (1). They showed that in patients with F3 fibrosis, vascular events and non-hepatic malignancies accounted for two-thirds of all major events (1). Similarly, Sanyal and colleagues showed that cardiovascular mortality was one of the most common causes of death in patients with non-alcoholic steatohepatitis (NASH) cirrhosis (27.6%), second only to infections (41.4%) (2).
Considering the prognostic impact of non-liver-related comorbidities in NAFLD/NASH, an integrated multidisciplinary approach is therefore a crucial model of care for these patients. Such a model was recently highlighted by Kumar and colleagues who described the ICHANGE model, which is tailored to individual patients with NAFLD (3). Although the overall clinical care is coordinated by a hepatologist, many other specialists are involved to face the various comorbidities associated with NAFLD: an endocrinologist, a dietitian and a cardiologist. Additional health providers, such as gastroenterologists, bariatric providers (surgical and endoscopic), and advanced practice providers, are involved in the care team when required.
The ICHANGE Model includes a referral algorithm for NAFLD starting with a liver screen to exclude other causes of liver disease, a clinical history, radiological investigations and the calculation of the Fibrosis-4 (FIB-4) score to risk stratify patients according to the presence of advanced fibrosis. This algorithm regroups patients into risk classes, and those classified as indeterminate or high risk for advanced fibrosis are referred to the ICHANGE providers. The patient journey starts with the explanation of the follow-up program and visit planning by a nurse, followed by a first visit with a hepatologist and a registered dietitian. The aim is to identify all associated comorbidities, evaluate the stage of liver disease, review lifestyle practices, and formulate treatment plans, firstly and mainly based upon 10% total body weight loss, as recommended in clinical practice guidelines (3,4). There are tailored consultations with a cardiologist or endocrinologist based on risk factors and perceived cardiovascular risk.
Strengths of such a model include the co-localization and co-ordination of care, with obvious advantages in quality of care, avoiding multiple hospital visits and promoting efficient communication both between healthcare professionals but also patients and practitioners. This results in reducing the risk of misdiagnosis, patient distrust and dissatisfaction and increases patient compliance. As a result, the risk of delaying diagnosis and therapies is overcome. Indeed, several studied that have evaluated MDCs have shown important improvements in patient-physician communication, better time and costs efficiency for clinicians and patients, improvements in quality-of-care and decrease in health care costs (5).
Some generic weaknesses in MDC models should be outlined. Patients are often required to go to a tertiary centre for consultations, thus forcing them to travel often for long distances. Moreover, this model of care requires that different specialists coordinate in the planning: if it is not possible for the patient to be seen simultaneously by several specialists, referrals and appointments must be organized and planned, consequently the number of days in which the patient should undergo specialist visits will increase, possibly decreasing the patient’s compliance. Last but not least, the collaboration from general practitioners is essential, in order to correctly stratify the risk of advanced fibrosis, with accurate history and laboratory investigations for valid screening with non-invasive tests. Specifically for the ICHANGE model, the inclusion of patients with indeterminate FIB-4 values has the risk of saturating capacity very quickly. Assuming a 5% prevalence of advanced fibrosis in an unselected population with NAFLD, 40% of patients would have an indeterminate or high FIB-4 and would require referral to the service. With increasing awareness of NAFLD and the availability of treatment in the next few years, it is unlikely that this strategy will be sustainable in the longer term. A refinement of the pathway with a second-tier non-invasive test such as Fibroscan or enhanced liver fibrosis (ELF) would overcome this weakness (6).
Overall, the benefits derived from MDC models of care like this one are indisputable. Firstly, the patient is followed and involved in the treatment process in dedicated and personalized plans, maximizing the clinical-diagnostic management of appointments and organizing subsequent follow-up visits in an integrated multidisciplinary way. This structured organization avoids long waiting times for visits and multiple referrals, uses resources in an efficient way and guarantees considerable economic savings to care facilities.
Furthermore, tele-health and in-person appointments can be staggered to improve patient compliance and assessment; this could be helpful also in those cases in which several specialists are not available in the same health facility, avoiding the patient to frequently move between clinics (3).
Other investigators tried to identify simple and effective guidelines for structuring the organization of similar models of care (see Table 1 for some examples of MDCs). In a recent review, Lazarus and colleagues looked for published examples of comprehensive models of care for NAFLD and the authors proposed some key recommendations, summarized in eight questions to be answered to organize a model of care in an optimal way and guide the practitioners and policymakers to improve assistance (14). These eight recommendations focus on what services should be provided and where, who should be the providers involved in the multidisciplinary clinic organization, and finally how these services should be integrated and coordinated. These recommendations can be helpful to ensure that care is delivered efficiently and effectively to NAFLD patients, maximizing cost-efficacy and sustainability of health systems in the management and follow-up of this complex disease.
Table 1
| Study | Population and period | Primary care | Secondary/tertiary care | Co-ordinator of the MDC model | Other health personnel part of the team or needed for further evaluation and follow-up |
|---|---|---|---|---|---|
| Cobbold et al. [2013] (7) | 180 patients, from 2007 to 2012 | Physicians assess risk of NAFLD: serum markers (e.g., ALT, AST, liver screen blood tests, NFS) are performed | NAFLD clinic provides personalized lifestyle counselling, medications to improve cardiovascular and diabetes risk and weight loss, and a 12-week supervised exercise program | Hepatologist | Diabetologist |
| Patient at-risk are referred via either hepatology services or diabetes services | Provisionally if required: cardiologist; bariatric provider | ||||
| Ahmed et al. [2017] (8) | NA | NA (not a referral clinic: patients seen directly in the tertiary centre as part of their HIV visit) | Metabolic clinic in the context of HIV clinic: assessing the risk of NAFLD | Metabolic medicine specialist | Infectious disease physician |
| Dietitian | |||||
| Armstrong et al. [2014] (9) | 95 new patient referrals, from 1 January 2010 to 31 December 2010 | Physicians assess the risk of NAFLD with liver function tests and/or abdominal US | Multidisciplinary NAFLD clinic: the hepatologist assesses the risk of NAFLD evolution and refers to other specialists if needed | Hepatologist | Endocrinologist |
| Diabetes specialist | |||||
| Dietitian | |||||
| Clinic research fellows | |||||
| Chalmers et al. [2020] (10) | 968 patients, from September 2016 to August 2017 | GPs assess the risk of liver diseases: AST/ALT ratio, FLI index | TE clinic: general anthropometric measures are taken, and a TE is performed | Hepatologist | GPs |
| From secondary care, some patient could be advised to follow local alcohol and/or weight management services in primary care | A brief lifestyle intervention guide is provided afterwards | Nurses | |||
| Health care-assistants trained to perform TE and deliver lifestyle interventions | |||||
| Eventually, if needed: cardiologist; dietitian; bariatric provider; endocrinologist | |||||
| Mantovani et al. [2020] (11) | 273 patients (no dates reported) | GPs assess a fibrosis risk evaluation with FIB-4 and ELF score | Multidisciplinary NAFLD clinic | Hepatologists | Dietitian |
| Cardiovascular expert | |||||
| Specialist nurse | |||||
| Moolla et al. [2019] (12) | 165 patients, from March 2014 to May 2017 | Risk stratification with NFS | Metabolic hepatology clinic | Hepatologists | Diabetologist, |
| Metabolic physician | |||||
| Specialist nurses | |||||
| Neilson et al. [2021] (13) | 50 consecutive patients attending hepatology clinics following implementation of the care bundle | Assessment of anthropometry, clinic evaluation for risk stratification | Multidisciplinary metabolic clinic | Hepatologists | Gastroenterologists |
| Specialist dietician | |||||
| Exercise physiotherapist | |||||
| Kumar et al. [2021] (3) | NA | Risk stratification and referral pathways. Assessment of FIB-4 and radiological imaging/clinical evidence | Multidisciplinary NAFLD clinic with in-situ and telehealth visits | Hepatologist | Nurse navigator |
| If both FIB-4 and TE are negative, the patient is followed in primary care, otherwise referred to NAFLD MDC | Registered dietician | ||||
| Endocrinologist | |||||
| Cardiologist | |||||
| Provisionally if required: endocrinologist; endoscopic bariatric provider; bariatric providers; gastroenterologist; advance practice providers |
NAFLD, non-alcoholic fatty liver disease; MDC, multi-disciplinary clinic; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NFS, NAFLD fibrosis score; NA, not available/applicable; HIV, human immunodeficiency virus; US, ultrasonography; GP, general practitioner; FLI, fatty liver index; TE, transient elastography; FIB-4, Fibrosis-4; ELF, enhanced liver fibrosis.
From our clinical experience, the application of these guideline in our MDCs has led to improvements in liver function tests, blood pressure, cholesterol levels, and glycated haemoglobin in diabetic patients (11); furthermore, the sequential use of non-invasive tests lowered secondary care referral rates (15). In our MDC model, the referral algorithm is based on a sequential use of FIB-4 and ELF score in primary care settings (see also Table 1). Following this algorithm, we achieved in a reduction of unnecessary referrals by 81% and a 5-fold increase in advanced fibrosis case detection (6).
In an evaluation of 273 patients with NAFLD, we demonstrated that a multidisciplinary approach in NAFLD was effective in improving liver-related and cardiovascular risk factors (11).
The above recommendation should be intended as a simple guide, to help healthcare and legislative personnel to improve assistance in NAFLD and other complex, pluri-comorbid diseases.
The ICHANGE Model is a good example of maximizing care with an efficient use of available resources, that translates into great benefits for the patients and economic savings for healthcare-facilities in the long term. Moreover, the implementation of an integrated care model can be leaner and more effective in clinical practice, thus providing great benefits also to the practitioners. The practitioners involved in organizing the MDC may vary, based on the context and healthcare system (see Table 1): a general example that can be adopted, showing the structure and practitioners involved in a NAFLD MDCs is shown in Figure 1.
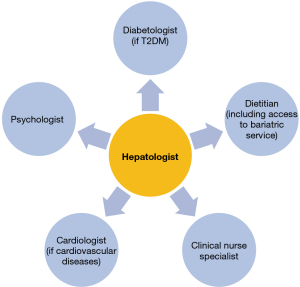
To conclude, we suggest that health systems should begin to implement MDC models in the management of patients with NAFLD. This will ensure the simultaneous optimal management of the liver disease component and the metabolic comorbidities, resulting in decreased morbidity and mortality. These models should be tailored to the individual health care systems to maximize efficiency.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-58/coif). EAT receives consulting fees as serving Advisory boards for Intercept, Pfizer, NovoNordisk unrelated to this work; and lecture fees from Intercept unrelated to this work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155:443-457.e17. [Crossref] [PubMed]
- Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682-9. [Crossref] [PubMed]
- Kumar S, Wong R, Newberry C, et al. Multidisciplinary Clinic Models: A Paradigm of Care for Management of NAFLD. Hepatology 2021;74:3472-8. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Gorin SS, Haggstrom D, Han PKJ, et al. Cancer Care Coordination: a Systematic Review and Meta-Analysis of Over 30 Years of Empirical Studies. Ann Behav Med 2017;51:532-46. [Crossref] [PubMed]
- Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol 2019;71:371-8. [Crossref] [PubMed]
- Cobbold JFL, Raveendran S, Peake CM, et al. Piloting a multidisciplinary clinic for the management of non-alcoholic fatty liver disease: initial 5-year experience. Frontline Gastroenterol 2013;4:263-9. [Crossref] [PubMed]
- Ahmed MH, Woodward C, Mital D. Metabolic clinic for individuals with HIV/AIDS: a commitment and vision to the future of HIV services. Cardiovasc Endocrinol 2017;6:109-12. [Crossref] [PubMed]
- Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM 2014;107:33-41. [Crossref] [PubMed]
- Chalmers J, Wilkes E, Harris R, et al. The Development and Implementation of a Commissioned Pathway for the Identification and Stratification of Liver Disease in the Community. Frontline Gastroenterol 2020;11:86-92. [Crossref] [PubMed]
- Mantovani A, Goyale A, Roccarina D, et al. A multidisciplinary approach to non-alcoholic fatty liver disease (NAFLD) improves cardiovascular risk factors. J Hepatol 2020;73:S110. [Crossref]
- Moolla A, Motohashi K, Marjot T, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol 2019;10:337-46. [Crossref] [PubMed]
- Neilson LJ, Macdougall L, Lee PS, et al. Implementation of a care bundle improves the management of patients with non-alcoholic fatty liver disease. Frontline Gastroenterol 2021;12:578-85. [Crossref] [PubMed]
- Lazarus JV, Anstee QM, Hagström H, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol 2021;18:717-29. [Crossref] [PubMed]
- Crossan C, Majumdar A, Srivastava A, et al. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: Diagnostic accuracy and cost analysis. Liver Int 2019;39:2052-60. [Crossref] [PubMed]


