An unusual case of adrenocortical carcinoma with liver metastasis that occurred at 23 years after surgery
Introduction
Adrenocortical carcinoma (ACC) is an uncommon and aggressive cancer occurring more frequently in women (sex ratio 1.5) (1). Two peaks of incidence are usually observed, between 1 and 6 years of age and during the fourth decade. Approximately 60% of ACC secrete hormones, with only 10% of them being malignant and usually associated with feminizing profile (2). Most of ACC cases are sporadic, but sometimes they could be associated with rare familial hereditary syndromes like Li-Fraumeni syndrome, Beckwith-Wiedmann syndrome, Multiple Endocrine Neoplasia I, Carney Syndrome and Lynch syndrome. Complete surgical resection currently remains the only potential curative treatment for ACC stages I-III, being and adjuvant administration of mitotane associated or not with systemic chemotherapy usually recommended due to the high risk of recurrence. Indeed, local or distant recurrence occur in 80% of cases after curative resection (3), mostly in liver, within a median delay of nine months (4). Here, we report the case of a unique liver metastasis from ACC occurring 23 years after the curative prior tumor surgery.
Case presentation
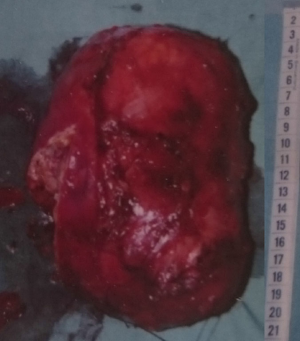
A 45-year-old woman was operated in 1991 for ACC without distant metastases. The initial surgery consisted on a left adrenalectomy with contemporaneous left nephrectomy and regional lymphadenectomy. Pathological findings revealed a 18 cm tumor (Figure 1): according to the current clinic-pathological and immune-histochemical parameters, the tumor was defined as an ACC (5). No microvascular involvement nor capsular infiltration were observed. No lymph node nor distant metastases were detected. At that time, no adjuvant treatment was indicated. Five years after surgery without sign of recurrence, the patient was considered cured.
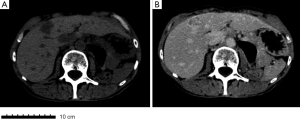
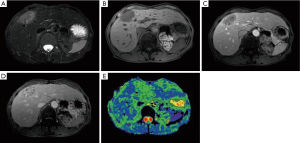
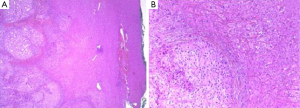
In 2014 (23 years later), the patient presented an atypical right subcostal pain. After a CT scan, a lesion mimicking the aspects of a hemangioma was observed (Figure 2). However, after a new evaluation of the images, a magnetic resonance imaging (MRI) was performed. The MRI revealed a 4-cm liver lesion involving the segment 4 (Figure 3), compatible with a liver metastasis. Percutaneous ultrasound-guided fine needle biopsy confirmed the suspect of an ACC. A curative resection of segment 4 was then performed. Final pathological examination further confirmed the diagnosis of an ACC metastasis with a complete R0 resection (Figure 4). Postoperative course was uneventful and the patient was discharged at day 7. No postoperative oncologic therapy was done, with the patient free from the tumor at 13 months after surgery.
Discussion
ACC is a rare malignancy with an incidence of 0.5 to 2 cases per million, being associated with a poor prognosis (6). Due to absence of specific symptoms, ACCs are frequently diagnosed at an advanced stage (III or IV) (3) or with distant metastases (7,8). Despite progress on systemic therapies, complete resection remains the only curative treatment (9).
The usual locations of ACC metastases are liver (47%), lungs (43%) and bones (25%) (10): unusual descripted locations have been stomach, skin and tongue (11). For liver metastasis, resection can be safely performed with acceptable postoperative morbidity. Indeed, Gaujoux et al. previously reported that resection of ACC liver metastases was an independent good predictive factor for overall survival, with a median survival of around 71.3 months compared to 18 months without surgery (12). Considering the management of the primary lesion, even in case of metastatic disease, surgery with chemotherapy or radiotherapy has been associated with a better prognosis (13). As reported by Ripley et al., recurrence after liver resections for ACC metastasis has been mostly reported at the level of the remnant liver after a median period of 11.4 months; a disease free interval (DFI) superior to 9 months has been associated with longer survivals (4). As previously reported by Datrice et al., repeated resections including major hepatectomy for recurrence could be justified: in fact, a better prognosis has been identified especially in patient with a DFI superior to 12 months, with a following median survival of 6.6 years after the recurrence resection, when compared to only 1.7 years if the DFI was initially inferior to 12 months (9). Concerning non resectable recurrent disease, trans-catheter arterial chemoembolization is an opportunity with interesting results (14).
The present case provides new information on the natural history of this rare tumor, in particularly regarding the possibility of a very late distant recurrence, even more than 20 years after the primitive tumor resection. Therefore, detection of single or multiple liver lesions in a patient considered in remission for an ACC might be first considered as a metastasis, and a fine needle biopsy should be immediately performed.
Interestingly, if we compare the present case to the one reported by Mawardi et al. (15), in both the cases the metastasis appeared tardily, with the primary tumor being a stage II lesion superior to 15 cm of diameter and without microvascular involvement or capsular infiltration. In these two cases, the main problem was the atypical clinical presentation and the initial doubtful diagnosis between an ACC metastasis and a primary liver tumor or an hemangioma.
Conclusions
We report here a case of a 68-year-old woman with a unique liver metastasis of ACC 23 years after the complete resection of the primitive stage II tumor. This metastasis represents the latest ever reported in literature for this type of tumor. Complete resection of the metastasis was conduct and to date, no recurrence was observed 13 months after resection. Past medical history must be never underestimated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 2006;91:2027-37. [Crossref] [PubMed]
- Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol 2008;190:1163-8. [Crossref] [PubMed]
- Fay AP, Elfiky A, Teló GH, et al. Adrenocortical carcinoma: the management of metastatic disease. Crit Rev Oncol Hematol 2014;92:123-32. [Crossref] [PubMed]
- Ripley RT, Kemp CD, Davis JL, et al. Liver resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol 2011;18:1972-9. [Crossref] [PubMed]
- Gurzu S, Jung I. Adrenocortical carcinoma: update of clinical features and diagnosis. Global Journal of Oncologist 2013;1:42-9.
- Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98:4551-64. [Crossref] [PubMed]
- Weiss LM, Medeiros LJ, Vickery AL Jr. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol 1989;13:202-6. [Crossref] [PubMed]
- Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol 1984;8:163-9. [Crossref] [PubMed]
- Datrice NM, Langan RC, Ripley RT, et al. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol 2012;105:709-13. [Crossref] [PubMed]
- Cuesta MA, Bonjer HJ, van Mourik JC. Endoscopic adrenalectomy: the adrenals under the scope? Clin Endocrinol (Oxf) 1996;44:349-51. [Crossref] [PubMed]
- Kovecsi A, Jung I, Bara T, et al. First Case Report of a Sporadic Adrenocortical Carcinoma With Gastric Metastasis and a Synchronous Gastrointestinal Stromal Tumor of the Stomach. Medicine (Baltimore) 2015;94:e1549.
- Gaujoux S, Al-Ahmadie H, Allen PJ, et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann Surg Oncol 2012;19:2643-51. [Crossref] [PubMed]
- Livhits M, Li N, Yeh MW, et al. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery 2014;156:1531-40; discussion 1540-1. [Crossref] [PubMed]
- Cazejust J, De Baère T, Auperin A, et al. Transcatheter arterial chemoembolization for liver metastases in patients with adrenocortical carcinoma. J Vasc Interv Radiol 2010;21:1527-32. [Crossref] [PubMed]
- Mawardi M, Al-Judaibi B, Marotta P. Hepatic metastasis from adrenocortical carcinoma fifteen years after primary resection. Saudi J Gastroenterol 2012;18:140-2. [Crossref] [PubMed]