Diagnosis and treatment of hepatic angiomyolipoma
Introduction
Hepatic angiomyolipoma (HAML) is a rare liver tumor that is characterized by its composition with blood vessel, smooth muscle and adipose tissue of varying proportions. Most of the HAML happens in adult females of Asian countries, such as China and Japan. The first case of HAML was reported by Ishak et al. in 1976 (1). Angiomyolipoma occurs more frequent in kidney. Liver ranks the second most frequent site. Misdiagnosis of HAML remains high because lack of variations in composition and radiological imaging findings (2). HAML has been considered as a benign disease; however, it was found recently that blood vessels and smooth muscles of HAML were monoclonal, while its lipid contents were polyclonal, suggesting that it is a true tumor. And some scholar proposed the possibility of malignant transformation (3-5). We performed a retrospective review on all HAML cases in our hospital in the past 8 years with the hope to summarize useful information for future clinical practice.
Materials and methods
Seventeen cases of HAML, which were treated in our department from November 2003 to June 2011, were reviewed retrospectively. Analysis was performed on various aspects of clinical manifestations including gender, age, clinical manifestations, imaging characteristics, intraoperative findings, and pathological features. All patients were followed up for recurrence and survival.
Results
Among the 17 patients, 13 were females and 4 were males (female: male = 3.25:1) with ages ranging from 27 to 60 with an average of 36.9 years old. Most of them were asymptomatic except that four patients had occasional right upper abdominal discomfort. Nine patients were diagnosed as HAML during routine physical examination or check-up for other diseases while the other eight were misdiagnosed. No obvious abnormalities were found from physical examination upon admission. Tumor markers AFP, CA19-9, CEA were within normal range in all patients (Table 1).
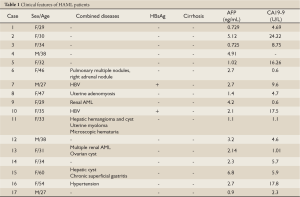
Full Table
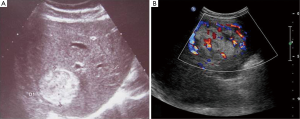
Thirteen patients went subjected to regular ultrasonography (US) examination. Two of them had lesions manifested as hypoechoic while the rest were middle or hyperechoic. The lesions were regular in shape with clear border (Figure 1). Blood flow signals were visible within or at peripheral of the lesions.
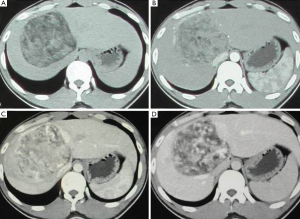
With computer tomography (CT) examination, all lesions showed clear boundary with surrounding tissue. Adipose tissue is visible inside lesion but different in size and range. In enhanced CT, the arterial phase was strengthened, but the enhancement demonstrated to be heterogeneous in each lesion and inconsistent in different patients. The enhancement has been weakened during the portal venous phase and delayed phase, and still be heterogeneous in nature (Figure 2).
Two patients underwent MRI and exhibited low signal in T1W1 and high signal in T2W1. Obvious enhancements were found in arterial phase, moderate enhancement in portal venous phase and delay phase as well.
One case was subjected to PET-CT examination for suspected atypical liver cancer. In 18F-FDG imaging the radioactive uptake was shown to be slightly reduced. With 11C-acetate, the early imaging of lesion on liver right lobe showed significantly higher perfusion; the delayed imaging of lesions showed uneven radioactive uptake increase, and SUVs 3.5-5.2.
All 17 patients underwent resection of liver tumor. The patient with both HAML and renal AML was subjected to two-way selective liver and kidney tumor embolization before surgery, which caused rupture of left kidney tumor and subsequent acute abdomen symptom. Emergency surgery was performed to remove both the left kidney and liver tumor. All the other patients had single mass in liver. Seven of them (41.2%) were on left lobe, 9 (52.9%) on right lobe and 1 (5.9%) on caudate lobe. Tumor size ranged from 1.6 cm × 1.0 cm to 17 cm × 10 cm. All patients recovered well after surgery.
Pathologic specimen showed all tumors but three having clean edge with surrounding tissue. The tumor exhibited gray-yellow, gray-red, or gray-brown in color, and soft or crisp in nature. Under the microscope, tumor was composed in different proportions of blood vessels, smooth muscle cells and adipose tissue (Figure 3A). In addition, one case showed large number of foam cells and one had extramedullary hematopoiesis.
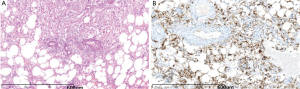
Immunohistochemistry staining was performed as follows: HBM45 (17/17) (Figure 3B), SMA (13/13) and CD34 (4/5), the S100 (4/7), Melan-A (3/3), MPO (1/2) were positive; CD117 (2/2), Heptocyte (6/6), AE1/AE3 (4/4), AFP (4/4), ER (1/1) were negative and Ki-67 index was about 5-10% in 5 cases.
All patients were followed and no tumor recurrence or any sign of metastasis has been found so far by US and CT examination.
Discussion
AML is more frequently found in kidney than in other organs such as liver and lung. While its tissue of origin is still not clear, HAML may be derived from perivascular epithelioid cells (PEC) (6), cells with multiple differentiation potentials that is capable of differentiating into the vascular smooth muscle and epithelial cell and expressing of the melanoma cell marker HMB-45 (7). This view has gradually gained attention among researchers that in the classification of soft tissue tumors in 2002 by the World Health Organization, HAML is classified as perivascular epithelioid cell tumors (PEComas) (8).
The particular tissue composition of HAML makes it hard to distinguish with other liver tumors. Correct preoperative diagnosis of HAML has been reported to be less than 25% (9,10). We were able to obtain a preoperative diagnostic rate of 53% (9/17), which is significantly higher from comprehensive analysis of clinical manifestations. Conventional imaging examinations such as US, CT, and MRL are the major preoperative diagnostic tools for HAML which shows distinctive imaging characteristics due to its content of adipose tissue, blood vessels and smooth muscle cells. However, since the histological compositions of HAML vary greatly among patients, the imaging variations of HAML can be in great deal, which is the main reason for misdiagnosis.
The relative typical appearance of HAML in ultrasonography imaging was regular round or oval mass with clear boundary. When the content of adipose tissue increases within the tumor, it shows a strong echo followed by attenuation. When the smooth muscle composition increases, it becomes hypoechoic with patchy or band of hyperechoic area, with no obvious attenuation. Increase of vascular ingredients within the tumor leads to hyperechoic that is similar to hemangioma (11). HAML appears as low-density lesions with clear boundary on CT scan. Typically, the adipose tissue appears as flaky or punctate distribution in the low-density environment and smooth muscle is shown as multinodular cystic. Dynamic contrast-enhanced scan for HAML shows that there is significant enhancement in the arterial phase, intratumoral blood vessels were visible. There is mild to moderate enhancement in the portal and delayed phases. Capsule degeneration and necrosis can also be found in some HAMLs (12). The characteristics of “fast in fast out” are similar to those of the hepatocellular carcinoma, which is the major reason for the misdiagnosis of HAML for HCC. The presence of adipose tissue is the key to distinguish HAML from HCC. Lipoma-like HAML is often misdiagnosed as lipoma or liposarcoma; while angiomatousis like HAML is often misdiagnosed as a hepatic hemangioma. MRI can be helpful for the differential diagnosis of HAML. Sakamoto (13) found that adipose tissue in HAML showed high signal on T1 weighted images, and the signal would be apparently different from that of adipose degeneration after continuously being strengthened for 6 min.
Final diagnosis of HAML depends on pathological examination. Cytology analysis using fine needle aspiration for suspected patients was an option (14), however, variations of the sites of needle aspiration may lead to different results due to the multiple components of HAML. HAML can be divided into four groups based on the tissue components and type of dominant tissue: (I) hybrid: typical and most common, contains similar proportions of each tissue components within the tumor; (II) myoma type: smooth muscle cells is the dominant tissue type within the tumor; (III) lipoma type: adipose tissue is the dominant tissue type; and (IV) hemangioma type: vascular tissue is the dominant type.
In addition to the microscopic morphology, immunohistochemistry staining can be used to improve the diagnostic specificity for HAML. HMB-45, a specific antibody against human melanocytic tumor, only reacts with HAML besides hepatoblastoma in liver tissue (15). Together with the expression of A103, SMA, and CD117, these markers play significant roles in the diagnosis of HAML.
As reported previously and same in our observation, HAML occurs mostly in females. However, this discrimination seems has little to do with sex hormone. Yeh et al. (15) concluded that sex hormones did not play a role in HAML development and HAML did not express estrogen or androgen receptors.
The onset of HAML is insidious; most patients have no obvious symptoms or signs, and are mostly discovered during routine physical examination. Some patients may feel discomfort in the upper abdominal region or pain in the liver. Only four patients in 17 reported here experienced intermittent discomfort at right upper abdomen, while the remaining had no obvious symptoms. Epigastric discomfort and other symptoms may be related to the increased tumor mass that leads to elevated tension in liver capsule and increased compression towards the surrounding tissue, etc. Although very rare, there were cases that patients came for treatment due to various complications such as aneurysm rupture, Budd-Chiari syndrome caused by compression of hepatic vein (16,17). One case of capsule rupture occurred in our cases that implicated the importance of early surgery to prevent it from happening.
HAML patients usually lack clear history of hepatitis; however, other types of liver lesions, such as liver hemangioma or liver cyst, were found in our cases (Table 1) and elsewhere (18). Several of our HAML patients also had chronic hepatitis B infection, adenomyosis, uterine fibroids, ovarian cysts, and intermittent microscopic hematuria. It is not yet clear whether or not these diseases are associated with HAML.
In conclusion, it is helpful to perform comprehensive analysis of US, CT, MRI imaging characteristics for correct preoperative diagnosis. Because of it malignant potential as well as the possibility of tumor rupture, surgical resection should be considered as early as possible.
Acknowledgements
We thank Haitao Zhao, Yiyao Xu, Haifeng Xu, Huayu Yang for helping collecting clinical images and data. We thank Xiangyang Liu for his help in preparation of this manuscript. This work is supported by the National Natural Science Foundation of China (No. 30901453).
Disclosure: The authors declare no conflict of interest.
References
- Ishak KG. Mesenchymal tumors of the liver. In: Okuda K, Peters RL. Hepatocellular Carcinoma. New York: John Wiley &Sons,1976:247-307.
- Zhong DR, Ji XL. Hepatic angiomyolipoma-misdiagnosis as hepatocellular carcinoma: A report of 14 cases. World J Gastroenterol 2000;6:608-12. [PubMed]
- Mizuguchi T, Katsuramaki T, Nobuoka T, et al. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol 2004;19:1328-30. [PubMed]
- Flemming P, Lehmann U, Becker T, et al. Common and epithelioid variants of hepatic angiomyolipoma exhibit clonal growth and share a distinctive immunophenotype. Hepatology 2000;32:213-7. [PubMed]
- Dalle I, Sciot R, de Vos R, et al. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology 2000;36:443-50. [PubMed]
- Folpe AL, Goodman ZD, Ishak KG, et al. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres: a novel member of the perivascular epithelioid clear cell family of tumors with a predilection for children and young adults. Am J Surg Pathol 2000;24:1239-46. [PubMed]
- Makhlouf HR, Ishak KG, Shekar R, et al. Melanoma markers in angiomyolipoma of the liver and kidney: a comparative study. Arch Pathol Lab Med 2002;126:49-55. [PubMed]
- Christopher DM, Fletcher K, Krishnan U, et al. Pathology and Genetics of Tumors of Soft Tissue and Bone. IARC Press, 2002:222.
- Yeh CN, Chen MF, Hung CF, et al. Angiomyolipoma of the liver. J Surg Oncol 2001;77:195-200. [PubMed]
- Chang Z, Zhang JM, Ying JQ, et al. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis 2011;20:65-9. [PubMed]
- Low SC, Peh WC, Muttarak M, et al. Imaging features of hepatic angiomyolipomas. J Med Imaging Radiat Oncol 2008;52:118-23. [PubMed]
- Yoshimura H, Murakami T, Kim T, et al. Angiomyolipoma of the liver with least amount of fat component: imaging features of CT, MR, and angiography. Abdom Imaging 2002;27:184-7. [PubMed]
- Sakamoto Y, Inoue K, Ohtomo K, et al. Magnetic resonance imaging of an angiomyolipoma of the liver. Abdom Imaging 1998;23:158-60. [PubMed]
- Al Nazer M, Ashraf MA. Fine-needle aspiration cytology of hepatic angiomyolipoma: case report with histological, immunohistochemical and electron microscopic findings. Ann Saudi Med 2001;21:324-33. [PubMed]
- Yeh CN, Lee KF, Chen MF. Immunohistochemical study of hepatic angiomyolipoma. Hepatogastroenterology 2005;52:1151-3. [PubMed]
- Guidi G, Catalano O, Rotondo A. Spontaneous rupture of a hepatic angiomyolipoma: CT findings and literature review. Eur Radiol 1997;7:335-7. [PubMed]
- Kelleher T, Staunton M, Malone D, et al. Budd Chiari syndrome associated with angiomyolipoma of the liver. J Hepatol 2004;40:1048-9. [PubMed]
- Tang LH, Hui P, Garcia-Tsao G, et al. Multiple angiomyolipomata of the liver: a case report. Mod Pathol 2002;15:167-71. [PubMed]


