Preoperative body mass index-to-prognostic nutritional index ratio predicts pancreatic fistula after pancreaticoduodenectomy
Introduction
Despite a marked reduction in mortality after pancreaticoduodenectomy, post-operative pancreatic fistula (POPF) remains one of the most common and serious complications. According to a large-scale national survey in Japan, grade B and C POPF defined by the International Study Group on Pancreatic Fistula (ISGPF) occurred in 13.2% of 8,575 patients undergoing pancreaticoduodenectomy (1). Although the majority of POPF can be managed by conservative therapy, some require reoperation (2). Furthermore, POPF leads to a prolonged hospital stay, substantial resource utilization, and sometimes to a life-threatening condition especially when associated with delayed massive bleeding (3,4).
Previous studies have identified a number of risk factors, most commonly intra- and post-operative variables, for POPF (5). For example, soft pancreatic texture assessed during surgery is known as the most commonly reported risk factor for POPF (6,7). Furthermore, drain amylase level measured postoperatively has been also reported to be useful in predicting the development of POPF (7,8).
Estimating the risk of POPF could improve patient risk stratification preoperatively and help with individualized patient consent. In this respect, preoperative factors are more useful than intra- or post-operative factors. Several preoperative risk factors for POPF after pancreaticoduodenectomy have been reported, including higher body mass index (BMI) and lower prognostic nutritional index (PNI) (9-11). There are, however, no simple and reliable preoperative predictors of POPF used currently in daily clinical practice. In the present study, we investigated the utility of BMI-to-PNI (BMI/PNI) ratio as a preoperative marker to predict the development of POPF in 87 patients undergoing pancreaticoduodenectomy.
Methods
Study subjects
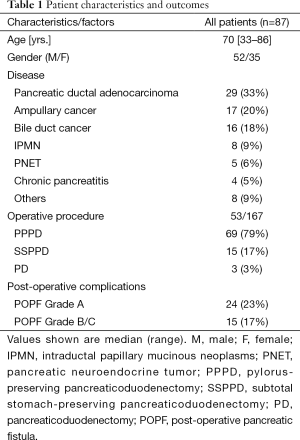
The study included a total of 87 consecutive patients underwent pancreaticoduodenectomy at our institution from May 2008 to March 2014. The patient characteristics are summarized in Table 1. There were 52 male and 35 female patients with a median age of 70 (range, 33−86) years. Indications for surgery included pancreatic ductal adenocarcinoma (29 patients), ampullary cancer (17 patients), bile duct cancer (16 patients), intraductal papillary mucinous neoplasm of the pancreas (eight patients), pancreatic neuroendocrine tumor (five patients), chronic pancreatitis (four patients), and other diseases. The operative procedures performed included pylorus-preserving pancreaticoduodenectomy (PPPD) in 69 patients, subtotal stomach-preserving pancreaticoduodenectomy (SSPPD) in 15 patients, and conventional Whipple operation (pancreaticoduodenectomy) in the remaining three patients.
Full table
Surgical techniques
Pancreaticoduodenectomy was done as previously described (12). Pancreaticojejunostomy was performed using a modified Kakita’s method (13). Adhesive sutures were placed through the pancreatic stump and run through the subserosal layer of the jejunum. A mucosa-to-mucosa anastomosis of the pancreaticojejunostomy was performed with interrupted sutures using 5−0 monofilament absorbable sutures (PDS, Ethicon Inc., Tokyo, Japan). A pancreatic duct drainage tube [a 4−6 Fr tube with an expanded segment selected according to the size of the main pancreatic duct (MPD)] was placed from the jejunal loop to the MPD. The pancreatic duct drainage tube was fixed at the anastomotic site using absorbable suture material and then externalized from a stab incision in the anterior abdominal wall. One or two intraabdominal drainage tubes were placed in the vicinity of the pancreaticojejunostomy.
Post-operative management and POPF
After surgery, all patients received intravenous antibiotics for three days [on the operation day and post-operative day (POD) 1 and 2]. In general, oral intake was gradually resumed around on day 3−5 if there was no evidence of intra-abdominal complications. The drain fluid amylase levels were measured on POD 3. The peripancreatic drains were removed usually on or after POD 7 if there was no evidence of leakage. When there was evidence of leakage or suspicion of an infectious complication, the peripancreatic drains were left and changed once a week.
The diagnosis of POPF was made by drain output of any measurable volume of fluid on or after POD 3 with amylase content 3 times greater than serum amylase activity, according to the International Study Group for Pancreatic Fistula (ISGPF) (14). In addition, the severity of POPF was graded as grade A, B, or C (14). In the present study, grade B and C POPF was defined as clinically relevant POPF.
Clinical variables for risk analysis of POPF
The medical records were reviewed for the following clinical variables: patient age, sex, BMI, PNI [calculated as 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3) (15)], serum albumin level and total lymphocyte count as components of PNI (but not used in the multivariate analysis), pancreatic gland texture (soft or hard), dilatation of the MPD at operation (5 mm or greater in diameter), operative time, intraoperative blood loss, drain amylase level on POD 3, and the volume of post-operative pancreatic juice output (during POD 1−3).
Data presentation and statistical analysis
The results of parametric data were expressed as medians (range). A univariate analysis of risk factors associated with POPF was performed using the Fisher’s exact probability test for categorical data and the Mann-Whitney U test for continuous data. A multivariate analysis was done for all variables with P-values of less than 0.2 using a logistic regression analysis. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were done using JMP 10 software (SAS Institute Inc., Cary, NC, USA).
Results
Post-operative pancreatic fistula (POPF)
Overall, POPF was diagnosed in 39 (45%) of 87 patients according to the ISGPF criteria. The severity of POPF was classified as grade A in 24 patents, grade B in 14, and grade C in 1 (Table 1). Therefore, clinically relevant (grade B/C) POPF occurred in 15 patients (17%). There was no 30-day mortality in this series.
Comparison of factors between patients with POPF and those without POPF
First, we compared various pre-, intra-, and post-operative variables between patients with POPF of all grades (n=39) and those without POPF (n=48). Univariate analysis revealed BMI (P=0.131), pancreatic consistency (P=0.0021), MPD dilatation (P=0.0060), and drain amylase level on POD3 (P<0.0001) to be significantly different between the groups. However, multivariate analysis including these significant variables failed to identify independent risk factors for POPF (data not shown).
Comparison of factors between patients with clinical POPF and those without clinical POPF
We then compared these variables between patients with clinical (grade B/C) POPF (POPF group, n=15) and those without clinical POPF (No-POPF group, n=72) (Table 2).

Full table
Among the preoperative factors, older age and male sex tended to be associated with the development of POPF, although the differences were not statistically significant. BMI was significantly higher in the POPF group than in the No-POPF group (23.3 vs. 21.2, P=0.0080). Preoperative PNI tended to be lower in the POPF group than in the No-POPF group (P=0.0533).
Of the intraoperative variables, a soft pancreatic texture was significantly more frequent in the POPF group than in the No-POPF group (100% vs. 64%, P=0.0040). There was no significant difference in the MPD dilatation (5 mm or greater), operative time, and intraoperative blood loss between the groups. With regard to the MPD dilatation, the difference was not significant (P=0.7625) even when the definition was changed to 3 mm or greater in diameter.
Regarding the post-operative variables, drain amylase level (on POD3) was significantly higher in the POPF group than in the No-POPF group (1,719 vs. 282 IU/L, P=0.0007). Furthermore, the volume of post-operative pancreatic juice output (during POD 1−3) was significantly larger in the POPF group than in the No-POPF group (276 vs. 151 mL, P=0.0206).
Multivariate analysis for factors associated with POPF
We then performed a multivariate analysis for factors predicting the development of clinical POPF using a logistic regression analysis. Because pancreatic consistency is assessed subjectively by the surgeon, this factor was excluded from the multivariate analysis. We also excluded drain amylase level because this consists of the definition of POPF. The multivariate analysis (including age, gender, BMI, PNI, and pancreatic juice output) revealed gender (male, P=0.0120), higher BMI (P<0.0001) and lower PNI (P=0.0251) to be independent factors associated with the development of clinical POPF (Table 3).

Full table
Analysis of BMI/PNI ratio as a preoperative predictor for POPF
We thought to determine if a combination of two independent factors, BMI and PNI, could be more useful in predicting the development of POPF. We therefore compared the BMI/PNI ratio between the POPF and No-POPF groups. The BMI/PNI ratio was significantly higher in the POPF group than in the No-POPF group (0.54 vs. 0.45, P=0.0007).
A receiver operating characteristic (ROC) curve analysis demonstrated a fair capability of BMI/PNI ratio to predict the occurrence of POPF (area under the ROC curve 0.781) (Figure 1). The area under the curve (AUC) of BMI/PNI ratio was higher as compared to that of BMI (0.719) or PNI (0.659) alone.
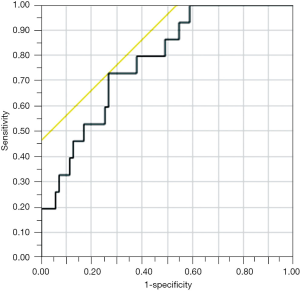
When a cut-off value of BMI/PNI ratio was set to 0.5, the sensitivity, specificity, and diagnostic accuracy to predict POPF was 73%, 74%, and 74%, respectively. In particular, when restricted to a subgroup of elderly (≥75 years old) male patients (n=10), the sensitivity, specificity, and diagnostic accuracy of BMI/PNI was 100%, 100%, and 100%, respectively.
Discussion
In this study, we retrospectively reviewed our series of 87 patients undergoing pancreaticoduodenectomy to identify risk factors for POPF. The major findings obtained were as follows: (I) the incidence of clinical POPF was 17%; (II) univariate analysis comparing patients with or without clinical POPF showed significant differences in BMI, pancreatic gland texture, drain amylase level, and the amount of pancreatic juice output; (III) multivariate analysis revealed a higher BMI and lower PNI to be independent risk factors for clinical POPF; (IV) BMI/PNI ratio was significantly higher in patients with POPF than in those without POPF; (V) a ROC curve analysis revealed AUC of BMI/PNI ratio to be 0.781; and (VI) the sensitivity, specificity, and diagnostic accuracy of BMI/PNI ratio to predict POPF was 73%, 74%, and 74%, respectively. These findings suggest that BMI/PNI ratio is a simple and reliable preoperative marker to predict the development of POPF after pancreaticoduodenectomy.
The reported risk factors for POPF after pancreaticoduodenectomy include preoperative variables (older age, male gender, a higher BMI, impaired preoperative nutritional status, type of disease, jaundice/biliary drainage, cholangitis, and normal exocrine function), intraoperative variables (soft pancreatic texture, small pancreatic duct, longer operative time, and increased blood loss during surgery), and post-operative variables (high amylase levels in the drain fluid and a larger volume of pancreatic juice output) (1,5-8,11,16-27). In addition to these individual risk factors, scoring systems combining several factors have been proposed to more accurately predict the risk of POPF (26,28). However, these scoring systems are sometimes too complex to use in daily clinical practice. In the present study, we combined only two preoperative factors, BMI and PNI, which were shown to be independently associated with POPF by multivariate analysis. Although high BMI and impaired preoperative nutritional status have been already recognized as risk factors for POPF (11), the clinical utility of BMI/PNI ratio has not been evaluated in previous studies. Because BMI and PNI can be obtained easily from physical examinations and basic laboratory data even at the first outpatient visit, BMI/PNI ratio may be a simple and useful preoperative marker for predicting the risk of POPF.
The strategy to decrease the incidence of POPF involves controlling its risk factors. In this respect, BMI/PNI can provide opportunities to select patients who could benefit from nutritional support. A previous study showed that preoperative immunonutrition decreases post-operative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreaticoduodenectomy (29). However, there is no sufficient evidence to support the efficacy of preoperative nutritional support to prevent POPF after pancreatectomy. Based on our present results, we introduced preoperative oral nutritional supplements (Ensure H, Abbott Japan) for a relatively short period (usually from the day of first visit to the day of surgery) in patients with a BMI/PNI ratio of >0.5. The efficacy of this preoperative nutritional support in patients at high risk for POPF should be prospectively evaluated in future studies.
In the present study, the sensitivity, specificity, and diagnostic accuracy of BMI/PNI ratio was 100%, 100%, and 100%, respectively, when restricted to a subgroup of elderly (≥75 years old) male patients. The reason why the diagnostic accuracy was particularly higher in this subgroup is unknown, but in general older age and male gender are also risk factors for POPF. Recently, increasing number of elderly patients become candidates for pancreaticoduodenectomy. Therefore, BMI/PNI ratio could be used to estimate the risk of POPF and help determine the indication of pancreaticoduodenectomy especially in elderly patients.
The present study has several limitations. First, this study is a retrospective analysis and the possibility of bias related to the historical background cannot be eliminated. Second, the number of patients (especially the number of patients who developed clinical POPF) in this study is relatively small and may be underpowered for some statistical analyses. To further validate our present findings, a prospective study in a larger number of patients is needed.
In summary, our present study demonstrates that BMI/PNI ratio is a simple preoperative marker to predict the occurrence of POPF after pancreaticoduodenectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee and written informed consent was obtained from all patients.
References
- Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773-80. [Crossref] [PubMed]
- Malleo G, Pulvirenti A, Marchegiani G, et al. Diagnosis and management of postoperative pancreatic fistula. Langenbecks Arch Surg 2014;399:801-10. [Crossref] [PubMed]
- Choi SH, Moon HJ, Heo JS, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004;199:186-91. [Crossref] [PubMed]
- Enestvedt CK, Diggs BS, Cassera MA, et al. Complications nearly double the cost of care after pancreaticoduodenectomy. Am J Surg 2012;204:332-8. [Crossref] [PubMed]
- Kawai M, Tani M, Hirono S, et al. How do we predict the clinically relevant pancreatic fistula after pancreaticoduodenectomy?--an analysis in 244 consecutive patients. World J Surg 2009;33:2670-8. [Crossref] [PubMed]
- Lin JW, Cameron JL, Yeo CJ, et al. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg 2004;8:951-9. [Crossref] [PubMed]
- Kawai M, Kondo S, Yamaue H, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:601-8. [Crossref] [PubMed]
- Molinari E, Bassi C, Salvia R, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg 2007;246:281-7. [Crossref] [PubMed]
- El Nakeeb A, Hamed H, Shehta A, et al. Impact of obesity on surgical outcomes post-pancreaticoduodenectomy: a case-control study. Int J Surg 2014;12:488-93. [Crossref] [PubMed]
- Del Chiaro M, Rangelova E, Ansorge C, et al. Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J Gastrointest Pathophysiol 2013;4:37-42. [Crossref] [PubMed]
- Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268-74. [Crossref] [PubMed]
- Yamaguchi K. Pancreatoduodenectomy for bile duct and ampullary cancer. J Hepatobiliary Pancreat Sci 2012;19:210-5. [Crossref] [PubMed]
- Kakita A, Yoshida M, Takahashi T. History of pancreaticojejunostomy in pancreaticoduodenectomy: development of a more reliable anastomosis technique. J Hepatobiliary Pancreat Surg 2001;8:230-7. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Schiesser M, Kirchhoff P, Müller MK, et al. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery 2009;145:519-26. [Crossref] [PubMed]
- Lerut JP, Gianello PR, Otte JB, et al. Pancreaticoduodenal resection. Surgical experience and evaluation of risk factors in 103 patients. Ann Surg 1984;199:432-7. [Crossref] [PubMed]
- Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg 1992;127:945-9; discussion 949-50. [Crossref] [PubMed]
- van Berge Henegouwen MI, De Wit LT, Van Gulik TM, et al. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 1997;185:18-24. [Crossref] [PubMed]
- Sato N, Yamaguchi K, Chijiiwa K, et al. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg 1998;133:1094-8. [Crossref] [PubMed]
- Sato N, Yamaguchi K, Yokohata K, et al. Preoperative exocrine pancreatic function predicts risk of leakage of pancreaticojejunostomy. Surgery 1998;124:871-6. [Crossref] [PubMed]
- Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery 2010;148:15-23. [Crossref] [PubMed]
- Tranchart H, Gaujoux S, Rebours V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg 2012;256:139-45. [Crossref] [PubMed]
- Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 2014;16:46-55. [Crossref] [PubMed]
- El Nakeeb A, Salah T, Sultan A, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg 2013;37:1405-18. [Crossref] [PubMed]
- Sugimoto M, Takahashi S, Gotohda N, et al. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 2013;17:1744-51. [Crossref] [PubMed]
- Roberts KJ, Sutcliffe RP, Marudanayagam R, et al. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg 2015;261:1191-7. [Crossref] [PubMed]
- Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008;32:419-28. [Crossref] [PubMed]
- Yamamoto Y, Sakamoto Y, Nara S, et al. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg 2011;35:2747-55. [Crossref] [PubMed]
- Aida T, Furukawa K, Suzuki D, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery 2014;155:124-33. [Crossref] [PubMed]


