
Propensity score matching study of 325 patients with spontaneous rupture of hepatocellular carcinoma
Introduction
Spontaneous rupture of hepatocellular carcinoma (HCC) is a dangerous bleeding complication of HCC. The incidence is 5–15% of patients with HCC in Asia and Africa where hepatitis B virus (HBV) infection is prevalent, but is rare in western countries (about 3%) (1,2). Although there are multiple methods to be chosen for the treatment including one-stage (emergency) hepatectomy, trans-arterial embolization (TAE), two-stage hepatectomy (following TAE) and conservative treatment, the study results with large sample, statistical matching and comparability data are rare, the survival assessment and optimal treatment remain controversial (3-6), doctors have to pursue the treatment options based on their own clinical experience so far.
Generally, compared with non-ruptured HCC (NHCC), larger tumor diameter and worse liver function are present in patients with ruptured HCC (RHCC), and the data of the two groups are not statistically comparable. This is the main reason for the controversial mentioned above.
In this study, the data from 2,616 patients with HCC were retrospectively studied with propensity score matching (PSM) method, for the purpose to properly assess the prognosis and to find out the possible optimal treatment for the complication. We present the following article in accordance with the TREND reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-45/rc).
Methods
The diagnosis of RHCC was established with the presence of hematoma, active peritumoral contrast extravasation on computed tomography, hepatic angiography on the first emergency admission, or with the evidence of tumor bleeding or peritumoral hematoma on surgery. TAE, one-stage (emergency) hepatectomy, two-stage hepatectomy and conservative treatment are the main therapeutic options for RHCC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the committee on Medical Ethics of the First Affiliated Hospital of Anhui Medical University (No. AF/SC-08/02.0). Individual consent for this retrospective analysis was waived.
Statistical analysis
Multivariate Cox proportional hazards model was used to analyze the risk factors related with the prognosis. Then, these covariates were matched by PSM with the allowable error at 0.3 (7-9) to compare the differences between the patients with RHCC and NHCC. The ratio of RHCC and NHCC patients was 1:4 in the PSM analysis. Nearest neighbor was the matching algorithm. The parameters included hemoglobin, leukocyte, albumin, aminotransferase, maximum tumor diameter, microvascular invasion and Child-Pugh grade were used as the matching variables in the PSM study. Independent t-test was used to analyze continuous data with a normal distribution which was tested by Kolmogorov-Smirnov test. Mann-Whitney U test was adopted for non-normal distributed data. Categorical variables were compared by the χ2 test with Yates’ correction or Fisher’s exact-test. Overall survival (OS) as well as disease-free survival (DFS) curves were generated with Kaplan-Meier method and compared by the log-rank test. The curves differences were examined with Cox proportional hazards analysis. Statistical analyses were performed with SPSS 23.0 program (SPSS Inc., Chicago, IL, USA). P<0.05 was considered as statistically significant.
Results
From January 2002 to June 2020, a total of 7,128 patients with HCC were admitted in Affiliated Hospitals of Anhui Medical University. Three hundred and sixty-six (5.1%) patients of them were diagnosed as spontaneous rupture of HCC with a hospital mortality of 0.8%. Complete follow-up information was harvested from 325 patients with RHCC and 2,291 patients with non-ruptured hepatocellular carcinoma. The median time of follow-up was 12 months (range, 1–202 months). The deadline of follow-up is June 30th, 2020. The parameter of patient with HCC was present in Table 1.
Table 1
| Variable | RHCC, n (%) | NHCC, n (%) | P value |
|---|---|---|---|
| Age | 54.5±13.8 | 56.0±12.5 | 0.091 |
| Sex | |||
| Male | 277 (85.2) | 1,974 (86.2) | 0.650 |
| Female | 48 (14.8) | 317 (13.8) | |
| AFP (µg/L), mean ± SD | 29,822.3±131,588.3 | 35,576.1±207,900.4 | |
| ≥400 | 170 (52.3) | 1,072 (46.8) | 0.062 |
| <400 | 155 (47.7) | 1,219 (53.2) | |
| Platelet (×109/L), mean ± SD | 176.8±90.0 | 172.4±100.9 | 0.487 |
| PT (s), mean ± SD | 13.5±3.3 | 13.2±3.3 | 0.099 |
| INR, mean ± SD | 1.16±0.36 | 1.04±1.05 | 0.779 |
| Hemoglobin (g/L), mean ± SD | 121.5±25.2 | 131.7±21.6 | <0.001 |
| Anemia | |||
| Yes | 142 (43.7) | 592 (25.8) | <0.001 |
| No | 183 (56.3) | 1,699 (74.2) | |
| Leukocyte (×109/L), mean ± SD | 8.4±5.4 | 6.7±4.4 | <0.001 |
| Albumin (g/L), mean ± SD | 36.6±7.1 | 39.2±10.1 | <0.001 |
| AST (U/L), mean ± SD | 113.8±159.3 | 90.2±133.9 | 0.018 |
| ALT (U/L), mean ± SD | 73.7±104.9 | 70.4±193.9 | 0.781 |
| TBL (µmol/L), mean ± SD | 24.5±49.5 | 26.2±52.8 | 0.646 |
| HBsAg | |||
| Positive | 279 (85.8) | 1,885 (82.3) | 0.111 |
| Negative | 46 (14.2) | 406 (17.7) | |
| Liver cirrhosis | |||
| Yes | 252 (77.5) | 1,994 (87.0) | <0.001 |
| No | 73 (22.5) | 297 (13.0) | |
| Child-Pugh classification | |||
| A | 224 (68.9) | 1,812 (79.1) | <0.001 |
| B | 83 (25.5) | 401 (17.5) | |
| C | 18 (5.5) | 78 (3.4) | |
| Maximum tumor diameter (cm) | Median [range]: 8 [1–30] | Median [range]: 6 [0.5–30] | |
| ≤2.9 | 6 (1.8) | 262 (11.4) | <0.001 |
| 3.0–5.0 | 48 (14.8) | 544 (23.7) | |
| ≥5.1 | 271 (83.4) | 1,485 (64.8) | |
| Microvascular invasion | |||
| Yes | 52 (16.0) | 202 (8.8) | <0.001 |
| No | 273 (84.0) | 2,089 (91.2) | |
| TNM stage | |||
| I | 96 (29.5) | 649 (28.3) | 0.110 |
| II | 110 (33.8) | 834 (36.4) | |
| III | 91 (28.0) | 534 (23.3) | |
| IV | 28 (8.6) | 274 (12.0) |
PSM, propensity score matching; RHCC, ruptured hepatocellular carcinoma; NHCC, non-ruptured hepatocellular carcinoma; AFP, alpha fetoprotein; SD, standard deviation; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; TBL, total bilirubin; TNM, tumor-node-metastasis.
Diagnosis
Sudden epigastric pain and shock were present in 146 (44.9%) and 43 (13.2%) patients with RHCC, respectively. The evidence of hematoma was found in 272 patients (83.7%) with imaging examination. Fifty-three (16.3%) patients were diagnosed during laparotomy.
Risk factors of RHCC
There were no significant differences in these variables including age, sex, alpha fetoprotein (AFP), platelet, prothrombin time (PT), international normalized ratio (INR), alanine transaminase (ALT), total bilirubin (TBL), HBsAg and tumor-node-metastasis (TNM) stage between RHCC and NHCC patients. But the parameters including maximum tumor diameter, microvascular invasion, leukocyte, aspartate aminotransferase (AST) and Child-Pugh grade were higher in RHCC than that in NHCC. These variables including hemoglobin, albumin and cirrhosis were lower in RHCC than that in NHCC (Table 1). The statistical difference mentioned above cannot be found after the PSM analysis (Table 2).
Table 2
| Variable | RHCC, n (%) | NHCC, n (%) | P value |
|---|---|---|---|
| Age | 54.8±13.9 | 54.9±12.8 | 0.880 |
| Sex | |||
| Male | 266 (85.0) | 917 (84.7) | 0.893 |
| Female | 47 (15.0) | 166 (15.3) | |
| AFP (µg/L) | |||
| ≥400 | 165 (52.7) | 560 (51.7) | 0.753 |
| <400 | 148 (47.3) | 523 (48.3) | |
| Platelet (×109/L), mean ± SD | 175.8±92.3 | 172.3±106.4 | 0.651 |
| PT (s), mean ± SD | 13.4±3.1 | 13.2±3.2 | 0.109 |
| INR, mean ± SD | 1.05±0.49 | 1.02±0.40 | 0.420 |
| Hemoglobin (g/L), mean ± SD | 121.8±26.1 | 122.5±28.8 | 0.745 |
| Anemia | |||
| Yes | 133 (42.5) | 400 (36.9) | 0.075 |
| No | 180 (57.5) | 683 (63.1) | |
| Leukocyte (×109/L), mean ± SD | 8.1±4.3 | 7.5±6.4 | 0.222 |
| Albumin (g/L), mean ± SD | 36.5±7.7 | 36.4±8.3 | 0.874 |
| AST (U/L), mean ± SD | 114.1±116.2 | 109.5±187.9 | 0.730 |
| ALT (U/L), mean ± SD | 74.2±106.4 | 75.7±119.9 | 0.862 |
| TBL (µmol/L), mean ± SD | 24.7±50.2 | 27.0±47.2 | 0.525 |
| HBsAg | |||
| Positive | 267 (85.3) | 915 (84.5) | 0.724 |
| Negative | 46 (14.7) | 168 (15.5) | |
| Liver cirrhosis | |||
| Yes | 247 (78.9) | 880 (81.3) | 0.355 |
| No | 66 (21.1) | 203 (18.7) | |
| Child-Pugh classification | |||
| A | 222 (70.9) | 791 (73.0) | 0.759 |
| B | 76 (24.3) | 245 (22.6) | |
| C | 15 (4.8) | 47 (4.3) | |
| Maximum tumor diameter (cm) | |||
| ≤2.9 | 6 (1.9) | 30 (2.8) | 0.618 |
| 3.0–5.0 | 47 (15.0) | 174 (16.1) | |
| ≥5.1 | 260 (83.1) | 879 (81.2) | |
| Microvascular invasion | |||
| Yes | 49 (15.7) | 135 (12.5) | 0.142 |
| No | 264 (84.3) | 948 (87.5) | |
| TNM stage | |||
| I | 88 (28.1) | 330 (28.0) | 0.629 |
| II | 107 (34.2) | 382 (35.3) | |
| III | 90 (28.8) | 280 (25.9) | |
| IV | 28 (8.9) | 118 (10.9) |
PSM, propensity score matching; RHCC, ruptured hepatocellular carcinoma; NHCC, non-ruptured hepatocellular carcinoma; AFP, alpha fetoprotein; SD, standard deviation; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; TBL, total bilirubin; TNM, tumor-node-metastasis.
Multivariate Cox analysis showed that the variables including age, AFP, hemoglobin, Child-Pugh classification, microvascular invasion and maximum tumor diameter were independently associated with the OS of RHCC. The risk of death for patients with RHCC who underwent one-stage hepatectomy is 1.545 times that of patients undergoing TAE + two-stage hepatectomy (Table 3).
Table 3
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.024 | 1.008–1.041 | 0.004 |
| Sex | 1.295 | 0.660–2.541 | 0.453 |
| AFP | 1.839 | 1.215–2.785 | 0.004 |
| PLT | 1.002 | 1.000–1.005 | 0.079 |
| PT | 0.944 | 0.860–1.036 | 0.224 |
| INR | 0.886 | 0.435–1.806 | 0.740 |
| Hemoglobin | 2.404 | 1.334–4.334 | 0.004 |
| Leukocyte | 0.938 | 0.879–1.001 | 0.053 |
| Albumin | 0.987 | 0.954–1.022 | 0.466 |
| AST | 1.001 | 0.999–1.003 | 0.268 |
| ALT | 1.000 | 0.998–1.003 | 0.754 |
| TBL | 1.010 | 0.997–1.023 | 0.131 |
| HBsAg | 1.048 | 0.952–1.154 | 0.339 |
| Liver cirrhosis | 1.406 | 0.873–2.266 | 0.161 |
| Child-Pugh classification | 2.428 | 1.569–3.756 | 0.000 |
| Maximum tumor diameter | 1.066 | 1.023–1.112 | 0.003 |
| Microvascular invasion | 1.815 | 1.140–2.890 | 0.012 |
| TNM stage | 1.069 | 0.895–2.890 | 0.459 |
| Main treatment | 1.545 | 1.120–2.132 | 0.008 |
CI, confidence interval; AFP, alpha fetoprotein; PLT, platelet; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine transaminase; TBL, total bilirubin; TNM, tumor-node-metastasis.
Pairing parameters in PSM study
Based on the difference between RHCC and NHCC, these parameters included hemoglobin, leukocyte, albumin, AST, maximum tumor diameter, microvascular invasion and Child-Pugh grade were used as the matching variables in the PSM study (Table 2).
OS
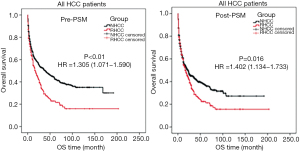
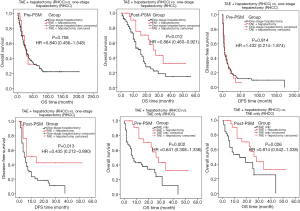
The hospital mortality of patients with RHCC was 0.9% (3 cases). Before or after the PSM analysis, the OS rate in patients with RHCC were significantly lower than that in patients with NHCC (P<0.05, Table 4, Figure 1). The median survival time was 17 months in patient with RHCC.
Table 4
| Treatment | Survival | PSM | Patients | N | OS or DFS rate (%) | Median OS or DFS (months) | P value | ||
|---|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | |||||||
| All kinds of treatment | OS | Before PSM | RHCC | 325 | 55.00 | 29.20 | 21.60 | 17 | <0.001 |
| NHCC | 2,291 | 64.20 | 50.90 | 42.80 | 40 | ||||
| After PSM | RHCC | 313 | 54.80 | 28.70 | 22.40 | 17 | 0.016 | ||
| NHCC | 108 | 57.20 | 43.10 | 37.00 | 23 | ||||
| Conservative treatment | OS | Before PSM | RHCC | 57 | 5.50 | 0 | 0 | 1 | <0.001 |
| NHCC | 520 | 17.90 | 6.50 | 3.60 | 3 | ||||
| After PSM | RHCC | 53 | 6.20 | 0 | 0 | 1 | 0.091 | ||
| NHCC | 158 | 7.00 | 0 | 0 | 2 | ||||
| TAE only | OS | Before PSM | RHCC | 69 | 43.20 | 12.20 | 0 | 7 | 0.569 |
| NHCC | 319 | 43.20 | 18.70 | 16.50 | 10 | ||||
| After PSM | RHCC | 41 | 34.00 | 5.70 | 0 | 6 | 0.233 | ||
| NHCC | 137 | 38.40 | 18.50 | 0 | 8 | ||||
| One-stage hepatectomy | OS | Before PSM | RHCC | 169 | 70.60 | 40.20 | 30.50 | 30 | <0.001 |
| NHCC | 1,452 | 82.80 | 69.50 | 58.40 | 101 | ||||
| After PSM | RHCC | 156 | 70.60 | 40.20 | 32.20 | 30 | <0.001 | ||
| NHCC | 624 | 78.70 | 63.90 | 50.60 | 63 | ||||
| DFS | Before PSM | RHCC | 169 | 34.60 | 16.40 | 12.00 | 6 | <0.001 | |
| NHCC | 1,452 | 55.20 | 38.50 | 28.20 | 17 | ||||
| After PSM | RHCC | 156 | 34.80 | 16.50 | 12.00 | 6 | <0.001 | ||
| NHCC | 624 | 48.70 | 34.50 | 24.10 | 12 | ||||
| TAE plus two-stage hepatectomy | OS | Before PSM | TAE + two-stage hepatectomy: (I) RHCC | 30 | 75.60 | 32.50 | 0 | 28 | (I) vs. (II), 0.758 |
| One-stage hepatectomy: (II) RHCC; (III) NHCC | 169; 1,452 | 70.6; 82.8 | 40.2; 69.5 | 30.5; 58.4 | 30; 101 | (I) vs. (III), 0.031 | |||
| TAE only: (IV) RHCC; (V) NHCC | 69; 319 | 43.2; 43.2 | 12.2; 18.7 | 0; 16.5 | 7; 10 | (I) vs. (IV), 0.002 | |||
| (I) vs. (V), 0.002 | |||||||||
| After PSM | TAE + two-stage hepatectomy: (I) RHCC | 28 | 75.60 | 32.50 | 0 | 28 | (I) vs. (II), 0.012 | ||
| One-stage hepatectomy: (II) RHCC | 82 | 53.10 | 14.80 | 0 | 16 | (I) vs. (III), 0.026 | |||
| TAE only: (III) RHCC | 33 | 53.40 | 25.30 | 0 | 14 | (I) vs. (IV), 0.485 | |||
| One-stage hepatectomy: (IV) NHCC | 100 | 76.70 | 58.60 | 0 | 37 | (I) vs. (V), 0.020 | |||
| TAE only: (V) NHCC | 65 | 40.10 | 0 | 0 | 11 | ||||
| DFS | Before PSM | TAE + two-stage hepatectomy: (I) RHCC | 30 | 38.10 | 6.30 | 0 | 10 | (I) vs. (II), 0.914 | |
| One-stage hepatectomy: (II) RHCC; (III) NHCC | 169; 1,452 | 34.6; 55.2 | 16.4; 38.5 | 12.0; 28.2 | 6; 17 | (I) vs. (III), 0.014 | |||
| After PSM | TAE + two-stage hepatectomy: (I) RHCC | 29 | 51.20 | 42.60 | 0 | 14 | (I) vs. (II), 0.013 | ||
| One-stage hepatectomy: (II) RHCC | 82 | 23.30 | 11.00 | 0 | 5 | (I) vs. (III), 0.221 | |||
| One-stage hepatectomy: (III) NHCC | 100 | 44.80 | 27.30 | 0 | 12 | ||||
PSM, propensity score matching; OS, overall survival; DFS, disease-free survival; RHCC, ruptured hepatocellular carcinoma; NHCC, non-ruptured hepatocellular carcinoma; TAE, trans-arterial embolization.
Since the different treatment results in different outcome, the patients were divided into conservative treatment, TAE and hepatectomy groups, to analyze the prognosis as following.
Conservative treatment
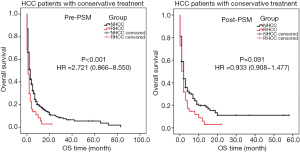
There are 57 (17.5%) RHCC patients and 520 (22.7%) NHCC patients underwent conservative treatments, with the median OS time of 1 and 3 months respectively. Two cases with RHCC died during hospitalization, one of them died of cardiac arrest and one died of upper gastrointestinal bleeding. After the PSM analysis, there was no significant difference of OS time between RHCC and NHCC patients (P=0.091, Table 4, Figure 2).
TAE only
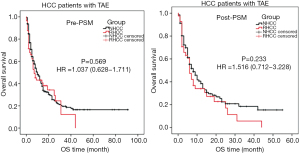
As the only treatment, TAE was carried out in 69 (21.2%) patients with RHCC and 319 (13.9%) NHCC cases, with the median OS of 7 and 10 months respectively. One patient with RHCC died after TAE treatment, the cause of death was liver failure. There was no significant difference between the two groups of patients in terms of OS (pre- and post-PSM study, P>0.05, Table 4, Figure 3).
One-stage hepatectomy
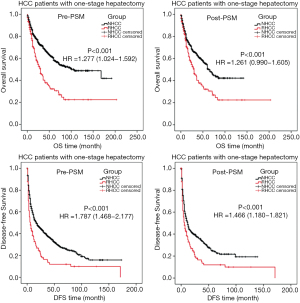
There are 169 (52.0%) RHCC cases and 1,452 (63.4%) NHCC cases underwent one-stage (emergency) open hepatectomy, with the median OS of 30 and 101 months respectively. Before and after the PSM study, the OS and DFS of RHCC patients were significantly shorter than that of NHCC (P<0.001, Table 4, Figure 4).
Two-stage hepatectomy
Since 1st January, 2015, a total of 30 RHCC patients have undergone emergency TAE and two-stage open hepatectomy, with the median OS and DFS time of 28 and 10 months respectively. The matching parameters were from NHCC patients who were hospitalized and underwent hepatectomy after 1st January, 2015.
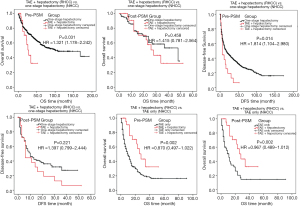
For RHCC patients, pre-PSM study result showed that there was no significant difference between the patients undergoing TAE + two-stage hepatectomy and the patients undergoing one-stage hepatectomy in terms of OS (P=0.758, Table 4). After PSM analysis, the OS and DFS time from two-stage hepatectomy were significantly longer than that from one-stage hepatectomy and TAE only (P<0.05, Table 4).
Compared with NHCC, although the pre-PSM OS was shorter in RHCC patients undergoing two-stage hepatectomy than that in NHCC patients undergoing hepatectomy (P=0.031, Table 4), post-PSM result showed that there was no significant difference between the two groups of patients in terms of OS and DFS (P=0.485, P=0.221, Table 4, Figures 5,6).
Discussion
It has been reported that tumor rupture can increase the rate of recurrence and reduce the survival rate (10,11). Tumor rupture was therefore overwhelmingly considered as the terminal status of HCC, for surgeons who might incline to passive attitude accordingly.
Although about 84% of the patients were diagnosed with RHCC before operation, 16% of them were diagnosed during laparotomy. To improve the preoperative diagnostic accuracy, abdominal puncture should be considered for these patients present with epigastric pain and anemia. The incidence of anemia is significantly higher in RHCC (43.7%) than that in NHCC (25.8%, P<0.001, Table 1).
It was found from this study, the median diameter of tumor is significantly larger in RHCC than that in NHCC (8 vs. 6 cm, P<0.001, Table 1). However, 54 patients had tumors less than 5 cm in diameter, and 6 of them were ≤2.9 cm (Table 1). There was no significant difference between small ruptured tumor (diameter ≤2.9 cm) and large reputed one with their clinical and pathological parameters. It means that the small tumors can also rupture. So, the positive therapies should be pursued.
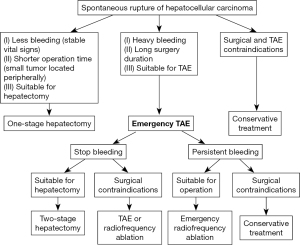
Currently, TAE is considered to be a preferable haemostasis option for RHCC patients, but with a poor prognosis (12,13). In this study, 21.2% [69] RHCC patients underwent TAE alone. Our data showed that there was no significant difference in OS time between RHCC and NHCC cases who underwent the treatment pre- and post-PSM analysis (Table 4, Figure 3). Our previous studies have found that, the injury and rupture of small artery in liver tissue may relate to the bleeding complication (14-17). With the embolization agents, TAE can block small artery (18,19), achieving dual effects of haemostasis and delaying tumor growth. Therefore, the same prognosis is present in RHCC and NHCC patients. It has been reported that the leading cause of death in the short-term was either bleeding complications or hepatic failure (20,21). Effective and minimally invasive emergent haemostasis such as TAE was recommended as the first choice with a successful haemostasis rate of 53–100% (3,22-24). The treatment of radiofrequency ablation should be considered if the bleeding persists after TAE treatment (Figure 7). Comfortingly, some of RHCC patients can then undergo two-stage hepatectomy after 2 weeks to 1 month of recovery.
Hepatectomy (laparotomy) is the main treatment for RHCC patient. Fifty-two percent [169] of RHCC patients underwent one-stage hepatectomy in this study. Although the dual purposes of haemostasis and tumor resection can be achieved by one-stage hepatectomy, the prognosis is poor. Pre- and post-PSM data showed that the OS and DFS were shorter in RHCC patient than that in NHCC patient (P<0.001, Table 4). The exacerbate liver damage caused by one-stage hepatectomy (secondary ischemia reperfusion injury) may be related to the poor prognosis (22,25). This result shows that one-stage hepatectomy may not be the best choice for patient with RHCC. However, it has been reported that there is no significant difference between 62 RHCC patients and 98 NHCC patients undergoing one-stage hepatectomy in terms of post-PSM OS (26). Beside the different number of enrolled patients, different criteria for patient selecting may also relate to the different results. It has been reported that minimally invasive treatment could be used for the tumor rupture (27-29) because it is able to alleviate the postoperative liver function. Although the data of long-term follow-up and large sample are absent, laparoscopic hepatectomy should be considered during emergency when the RHCC patient with less bleeding (stable vital signs) and small tumor located peripherally, since the treatment with a minimal invasion and shorter operation time can maximize the benefit of the patient. It is thought that TAE followed by two-stage hepatectomy is a better option for patient with RHCC (25). In this study, 30 cases with RHCC underwent TAE + two-stage hepatectomy. For RHCC patient, pre-PSM result showed that there was no difference between TAE + two-stage hepatectomy and one-stage hepatectomy outcomes in terms of OS and DFS (P=0.758, P=0.914, Table 4). This may lead to a misunderstanding: for patients with RHCC, the treatment outcome of one-stage hepatectomy is the same as that of two-stage hepatectomy, so the one-stage hepatectomy is accepted as the main therapy. Post-PSM result showed that the outcome of TAE + two-stage hepatectomy was much better than that of one-stage hepatectomy (Table 4). The different criteria to select patients of the two kinds of treatments may result in the statistical deviation.
The post-PSM results in this study showed that the treatment outcomes from TAE + two-stage hepatectomy for RHCC patients is the same as that from hepatectomy for NHCC patients; the outcomes are better than that from other therapies including one-stage hepatectomy and TAE alone for patient with RHCC. These results indicate that: (I) TAE + two-stage hepatectomy might be the optimal choice for patient with RHCC if the tumor is resectable; (II) the incidence of tumor recurrence and tumor cells spread may not increase with the tumor rupture, since the significant difference of DFS between RHCC and NHCC patients cannot be found. This result may relate to our following surgical procedure: after the tumor is removed, a large amount of warm normal saline (>10 L, 30 ℃) is used to wash the abdominal and pelvic cavity until the aspirated wash fluid is completely clear, in order to avoid the implantation of tumor cells. Our results are consistent with Zhou’s study on 102 RHCC patients (30). Though it has been reported that more incidence of tumor recurrence was secondary to the tumor rupture (10), our pre-PSM data showed that incidence of microvascular invasion and TNM stage were higher in RHCC patient than that in NHCC patient. The pathological properties of the tumor may result in the poor prognosis of patient with RHCC. Under the premise of the same pathological properties, there is no difference of the treatment outcome between RHCC patients and NHCC patients if the therapy is appropriate.
In this study, 52% RHCC patients underwent one-stage hepatectomy with a poorer prognosis, which may result in the shorter OS from all of the RHCC patients than that of the NHCC patients (P=0.016, Table 4). These variables including age, AFP, hemoglobin, Child-Pugh classification, microvascular invasion and maximum tumor diameter were independent factors associated with the OS of RHCC (Table 3). The risk of death for RHCC patients undergoing one-stage hepatectomy is 1.545 times higher than that of patients undergoing TAE + two-stage hepatectomy (P=0.008, Table 3).
In this study, a total of 57 (17.5%) RHCC patients underwent conservative treatment, with the median OS time of 1 month. The post-PSM analysis result showed that, there is no significant difference between RHCC and NHCC patient undergoing conservative treatment in terms of OS, which reveals that the tumor rupture itself may not aggravate the progression of the disease.
For patient with RHCC, TAE followed by two-stage hepatectomy might be the optimal choice, which OS and DFS is no significant difference compared with NHCC patient undergoing hepatectomy, and with a better outcome than that with other kinds of treatment such as TAE alone or one-stage hepatectomy.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (grant numbers: 51872279 and 52072005).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-45/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-45/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the committee on Medical Ethics of the First Affiliated Hospital of Anhui Medical University (No. AF/SC-08/02.0). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Yang T, Sun YF, Zhang J, et al. Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg 2013;100:1071-9. [Crossref] [PubMed]
- Kung CT, Liu BM, Ng SH, et al. Transcatheter arterial embolization in the emergency department for hemodynamic instability due to ruptured hepatocellular carcinoma: analysis of 167 cases. AJR Am J Roentgenol 2008;191:W231-9. [Crossref] [PubMed]
- Leung KL, Lau WY, Lai PB, et al. Spontaneous rupture of hepatocellular carcinoma: conservative management and selective intervention. Arch Surg 1999;134:1103-7. [Crossref] [PubMed]
- Sada H, Ohira M, Kobayashi T, et al. An Analysis of Surgical Treatment for the Spontaneous Rupture of Hepatocellular Carcinoma. Dig Surg 2016;33:43-50. [Crossref] [PubMed]
- Chan AC, Dai JW, Chok KS, et al. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery 2016;159:409-17. [Crossref] [PubMed]
- Sakamoto T, Fujiogi M, Lefor AK, et al. Stent as a bridge to surgery or immediate colectomy for malignant right colonic obstruction: propensity-scored, national database study. Br J Surg 2020;107:1354-62. [Crossref] [PubMed]
- Amelung FJ, Borstlap WAA, Consten ECJ, et al. Propensity score-matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg 2019;106:1075-86. [Crossref] [PubMed]
- Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996;52:249-64. [Crossref] [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol 2001;19:3725-32. [Crossref] [PubMed]
- Uchiyama H, Minagawa R, Itoh S, et al. Favorable Outcomes of Hepatectomy for Ruptured Hepatocellular Carcinoma: Retrospective Analysis of Primary R0-Hepatectomized Patients. Anticancer Res 2016;36:379-85. [PubMed]
- Shinmura K, Choi YH, Shimohira M, et al. Comparison of conservative treatment versus transcatheter arterial embolisation for the treatment of spontaneously ruptured hepatocellular carcinoma. Pol J Radiol 2018;83:e311-8. [Crossref] [PubMed]
- Zou J, Li C, Chen Y, et al. Retrospective analysis of transcatheter arterial chemoembolization treatment for spontaneously ruptured hepatocellular carcinoma. Oncol Lett 2019;18:6423-30. [Crossref] [PubMed]
- Zhu LX, Geng XP, Fan ST. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg 2001;136:682-7. [Crossref] [PubMed]
- Zhu LX, Liu Y, Fan ST. Ultrastructural study of the vascular endothelium of patients with spontaneous rupture of hepatocellular carcinoma. Asian J Surg 2002;25:157-62. [Crossref] [PubMed]
- Zhu LX, Meng XL, Fan ST. Elasticity of small artery in patient with spontaneous rupture of hepatocellular carcinoma. Hepatol Res 2004;29:13-7. [Crossref] [PubMed]
- Zhu LX, Xu XL, Geng XP, et al. Circumferential stress on blood vessel and its application. Journal of Medical Biomechanics 2004;19:160-5.
- Ngan H, Lai CL, Fan ST, et al. Transcatheter arterial chemoembolization in inoperable hepatocellular carcinoma: four-year follow-up. J Vasc Interv Radiol 1996;7:419-25. [Crossref] [PubMed]
- Firouznia K, Ghanaati H, Alavian SM, et al. Transcatheter arterial chemoembolization therapy for patients with unresectable hepatocellular carcinoma. Hepat Mon 2014;14:e25792. [Crossref] [PubMed]
- Moris D, Chakedis J, Sun SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: A systematic review. J Surg Oncol 2018;117:341-53. [Crossref] [PubMed]
- Tanaka S, Kaibori M, Ueno M, et al. Surgical Outcomes for the Ruptured Hepatocellular Carcinoma: Multicenter Analysis with a Case-Controlled Study. J Gastrointest Surg 2016;20:2021-34. [Crossref] [PubMed]
- Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg 2006;141:191-8. [Crossref] [PubMed]
- Yoshida H, Mamada Y, Taniai N, et al. Spontaneous ruptured hepatocellular carcinoma. Hepatol Res 2016;46:13-21. [Crossref] [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol 2001;19:3725-32. [Crossref] [PubMed]
- Zhu LX, Wang GS, Fan ST. Spontaneous rupture of hepatocellular carcinoma. Br J Surg 1996;83:602-7. [Crossref] [PubMed]
- Chua DW, Koh YX, Allen JC, et al. Impact of spontaneous rupture on the survival outcomes after liver resection for hepatocellular carcinoma: A propensity matched analysis comparing ruptured versus non-ruptured tumors. Eur J Surg Oncol 2019;45:1652-9. [Crossref] [PubMed]
- Khairuddin A, Ong GH, Tan JS, et al. Emergency laparoscopic resection of spontaneous rupture of hepatocellular carcinoma: A case report. Int J Surg Case Rep 2020;66:104-6. [Crossref] [PubMed]
- Yoshiya S, Iwaki K, Sakai A, et al. Laparoscopic Left Hepatectomy for Ruptured Hepatocellular Carcinoma Controlled After Transcatheter Arterial Embolization: Case Report and Review of the Literature. In Vivo 2018;32:659-62. [PubMed]
- Kabir T, Tan ZZX, Chua DW, et al. Early experience with laparoscopic liver resection for spontaneously ruptured hepatocellular carcinoma. J Minim Access Surg 2020;16:239-45. [Crossref] [PubMed]
- Zhou C, Zhang C, Zu QQ, et al. Emergency transarterial embolization followed by staged hepatectomy versus emergency hepatectomy for ruptured hepatocellular carcinoma: a single-center, propensity score matched analysis. Jpn J Radiol 2020;38:1090-8. [Crossref] [PubMed]


