Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology
Hepatic stellate cells (HSCs) and liver immunity
The liver is an immunologic organ consisting of parenchymal cells (i.e., hepatocytes and cholangiocytes), non-parenchymal cells [e.g., endothelial cells, Kupffer cells (KC), biliary cells, HSC] and manifold cell types from the innate and adaptive immune system. From all these cell fractions, the precise role of HSC in normal liver is far less understood compared to other hepatic cell entities. Although it is well accepted that HSC can very dynamically adapt their phenotype and are a major contributor to the formation of experimental and human fibrosis (1-3), the exact function of HSC in normal liver is still controversially discussed. They are defined as fat-storing pericytes that constitute 5-8% of all liver cells (4). HSC contain approximately 80% of the body’s vitamin A (Vit A) with a gradual distribution in the liver lobules that depends on the total Vit A amount and is genetically determined (5,6).
During the last decades, HSC were mainly viewed as a “resting cell” that stores Vit A, impacts sinusoidal blood flow, mediates intercellular communication, synthesises various extracellular matrix components, and triggers the synthesis of polypeptide mediators, erythropoietin and components of the plasminogen activation system that guarantee homeostasis in the microenvironment of the hepatic sinusoid (1,3,7). For instance, HSC can produce erythropoietin (8), a hematopoietic growth factor with potentially beneficial actions during liver regeneration or recovery from injury (9-11).
However, the repertoire of HSC functions was recently found to be much more diverse, as it was reported that they can act as antigen presenting cells (APC), express pattern recognition receptors (PRRs), respond to damage associated molecular patterns (DAMPs), and have the capacity to interact with manifold immune cells, and modulate their activity or promoting their differentiation (Figure 1). For instance, HSC possess profound T cell inhibitory activity in vitro and the activation of HSC is associated with enhanced expression of B7-H1 [CD274, programmed death-ligand 1 (PD-L1)] that plays a major role in suppressing adaptive immune responses (12). Interestingly, quiescent HSC do not express this inhibitory transmembrane protein and it can be markedly up-regulated after activation with interferon-γ (IFN-γ) or contact with activated T cells. Extension of this work has further demonstrated that HSC effectively protected islet allografts from rejection in an islet transplantation model (13). Moreover, HSC interact with immune cells in a bidirectional manner (14). They receive a plenitude of signals from individual immune cells and in turn produce many soluble inflammatory mediators that elaborate signals influencing the biological properties of different immune cells. Important signalling pathways for HSC activation include, for example, the nuclear factor kappa B (NF-κB) that is involved in HSC activation upon lipopolysaccharide (LPS) or TLR4 stimulation or ATP-induced cytosolic Ca2+ influx via purinergic signalling receptors including P2Y (15). During phases of hepatic insult, HSC produce reactive oxygen species (ROS), pro-inflammatory cytokines, chemokines and their receptors and can act as non-professional APCs (1,7,16). On the other hand, HSC depletion experiments revealed that shortage in HSC is associated with elevated expression of interleukin 10 (IL-10) and IFN-γ and that activated HSC significantly amplify the response to liver injury (17).
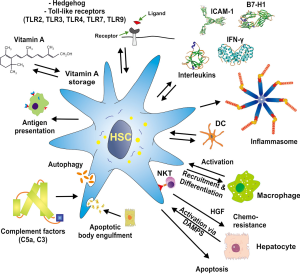
All these studies suggest that HSC significantly contribute to and participate in liver immunity. In the present review, we will summarize the major features of HSC and their interference with other liver resident and infiltrating cell entities that have established them as an immune-modulatory cell with key functions in liver immunology.
Vit A and the immune system
The fat soluble Vit A (retinol) and its derivative retinoic acid have pleiotropic functions in immune responses and liver homeostasis (Figure 2). It is one of the vitamins that cause disease not only as the result of deficient but also of excessive intake (18). Experimentally, it was demonstrated that there is a close correlation between (I) liver damage and decrease in retinaldehyde and total retinol; (II) a reciprocal transfer of retinoid metabolites between the circulation and the liver during development of cirrhosis; and (III) Vit A deprivation and liver parenchyma injury (19,20). However, also severe hyperalimentation with Vit A dose-dependently results in perisinusoidal fibrosis (21). The majority of the total body Vit A is stored in the liver, and 80-90% of the retinoid in the liver are stored in the lipid vacuoles of HSC (22-24). Whether Vit A directly influences pro- or antifibrogenic functions of HSC is still under discussion in the field. In lecithin-retinol acyltransferase (LRAT)-deficient mice, which lack retinoid-containing lipids droplets in their HSCs, experimental fibrosis development was unaltered, suggesting that absence of retinoid-containing HSC lipid droplets does not promote HSC activation (25).
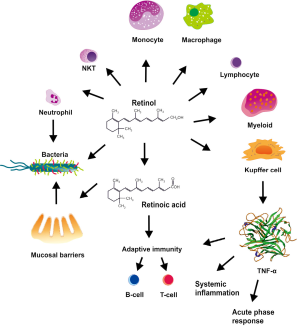
There a several studies revealing an eminent role of Vit A in innate immunity and adaptive immune functions. Vit A regulates innate immunity by impeding normal regeneration of mucosal barriers, diminishing or enhancing the functions of neutrophils, monocytes, macrophages, natural killer (NK) cells, lymphocyte, and modulating myeloid cell differentiation (26-33). Vit A appears of particular relevance for the differentiation of CD11b+ dendritic cell (DC) subsets in the mucosa as well as the spleen, which are important for anti-bacterial and anti-fungal immune responses as well as for mucosal immunity (34). Moreover, Vit A is required for adaptive immunity and evolves essential functions in the development and activation of T-helper and B-cells including antibody-mediated responses directed by Th2 cells (25,35). Interestingly, Vit A is already important in the embryogenic development of the immune system, with retinoic acid supplied via the maternal blood being an important trigger for distinct lymphoid cells and subsequent functionally competent immune structures in the offspring (36).
When rats are treated with high concentrations of Vit A for 3-7 days, the phagocytic activity of KC and peripheral blood monocytes markedly increase, and the basal production of tumor necrosis factor-α (TNF-α) and prostaglandin E2 are significantly elevated (37). Similarly, it was demonstrated that Vit A supplementation in Vit A-deficient rats restores neutrophil function and trigger the ability of the neutrophils to phagocytose common bacteria (e.g., Pseudomonas aeruginosa) and to produce active oxidant molecules (28). At the molecular level, it has been demonstrated that Vit A suppresses the production of nitric oxide (NO) and pro-inflammatory cytokines (e.g., TNF-α, IL-1β) in LPS- or IFN-γ-stimulated macrophages (38,39). In line with all these experimental studies, Vit A supplementation in infectious diseases reduces morbidity and mortality in humans (40). The positive attributes of Vit A were also noted in a small cohort of volunteers with low Vit A stores that were supplemented with Vit A resulting in higher concentrations of NK cells, elevated concentrations of monocyte-derived ROS, higher serum concentration of CXCL10 (IP10), and lower IL-6 and IL-17 serum concentrations (41). The direct regulatory effect of retinoic acid on natural killer T (NKT) cells was further demonstrated in a model of concanavalin A-induced hepatitis in mice showing that retinoic acid differentially regulates the secretion of various pathogenic cytokines from NKT cells (42). During the last years, the fundamental knowledge how Vit A acts mechanistically in HSC increased dramatically. In the presence of all-trans retinoic acid and TGF-β1, HSC induce the expression of FoxP3 in CD4 positive T cells, thereby playing an essential role in the tolerogenic nature of the liver (43,44).
Complement factors and stellate cells
The family of about 30 soluble complement proteins are synthesized both in the liver for systemic release into the bloodstream and peripherally by tissue-resident and immune cells (45). The complement component 5 is composed of two polypeptide chains (C5a and C5b) that are linked by disulfide bridges and are released by limited proteolysis during complement activation. The G-protein-coupled C5a receptor (C5aR) that reduces suppressive activity of natural regulatory T cells (Treg) is expressed on KC, HSC, and weakly on sinusoidal endothelial cells (45-47). With regard to liver, it was demonstrated that the C5 gene in mice and humans is a confounding factor that contributes to the formation of hepatic fibrosis (48). Likewise, the C5a/C5aR axis was shown to mediate inflammatory, chemotactic and anaphylatoxic properties in innate and adaptive immunity as well as to modulate activation and migration of HSC (49,50). Several other complement factors including C3 and C4 were shown to correlate negatively with the Child-Pugh score in patients suffering from liver cirrhosis (51). HSC express C5aR and upon stimulation with C5a upregulate fibronectin expression and release prostanoids (46). Moreover, other studies have shown that HSC express the complement-activating protease P100, while other cells of the hepatic sinusoid including KC and sinusoidal endothelial cells are negative for this key complement activator (52). The same study demonstrated that the expression of this selene protease is elevated during phases of liver insult and the acute phase response suggesting that complement activation is one essential mechanism of hepatic innate immunity (52). Interestingly, also the C4 complement was shown to be expressed by HSC with a biphasic expression profile, in which freshly isolated HSC showed minimal expression of C4 mRNA that decreased between days 3 and 7 of primary culture and were absent in passaged, fully transdifferentiated myofibroblasts (MFB) (53).
Immune-associated signalling in HSC
Hedgehog (Hh) signalling
The three Hh proteins [Sonic Hh (Shh); Indian Hh (Ihh); Desert Hh (Dhh)] and their pathways have remarkable biological importance in control of progenitor cell fate and during embryogenesis (54). In the adult liver, Hh signalling pathways can be reactivated during liver injury and mediate expansion and recruitment of liver progenitor cell populations, induce the production of pro-fibrogenic factors such as IL-4 and IL-13, regulates pro-survival pathways and drives hepatic accumulation of inflammatory cells (55). First reports showing that Hh signaling could possibly be important in HSC biology were published about one decade ago, when Sicklick and co-workers found that freshly isolated HSC from adult mice contained transcripts of Hh ligands, the Hh pathway receptor, and Hh-regulated transcription factors in mice, rats and humans (56). In transwell co-cultures, it was shown that HSC-derived Shh is capable to modulate proliferation and differentiation of marrow-derived mesenchymal stem cells (57). Likewise, it was demonstrated that an immortal human HSC cell line (i.e., LX-2) stimulated proliferation, migration and invasion of cholangiocarcinoma in an Hh-dependent manner demonstrating that targeting the Hh pathway could be a promising therapeutic direction (58). A detailed gene expression analysis also confirmed that Hh signaling is necessary to confer sensitivity to systemic LPS challenge and that mice with reduced receptors for Shh are more resistant to LPS than control mice (59). Recently, studies conducted in lung tissue have shown that upregulated Hh proteins in lung epithelial cells during allergic inflammation modulate lymphocyte function and interfere with T cell differentiation (60). It is presently not known if the quantities of Hh secreted from HSC during hepatic inflammation are sufficient to modulate local T cell functions as well.
TLR signalling
Most cells that are involved in the defense against pathogens have specialized heterologous receptors, namely the PRRs that recognize specific pathogen associated molecular patterns (PAMPs) and respond to endogenous triggers that are classified as DAMPs. TLRs, for example, are one group of 10 proteins in humans (TLR1-10) and 12 in mice (TLR1-9 and TLR11-13) that recognize and sensitize for structurally conserved molecules derived from microbes. Since the liver is permanently exposed to many pathogens and commensally bacterial products vial the portal vein it is not surprising that many liver cells express TLRs (61). Most of the TLRs form homodimers, while TLR2 forms heterodimers with TLR1 or TLR6 influencing the specificity for certain ligands. The complexity of this system is further increased by variable affinities for additional co-receptors that are sometimes required to obtain full ligand sensitivity. Prototypically, this has been demonstrated for TLR4 which require the lymphocyte antigen 96 also known as MD-2 that confers LPS responsiveness on TLR4 (62). Other proteins such as the PPR CD14 and the LPS-binding protein (LPB) are further necessary to facilitate the presentation of LPS to MD-2 and to finally activate TLR4 (63).
Once activated, the TLRs signal through a complex network of at least two different pathways that are characterized by different adaptor proteins. One pathway that is common to all TLRs (except TLR3) requires the myeloid differentiation factor 88 (MyD88), Toll/IL-1 receptor domain containing adapter protein (TIRAP), Toll/IL-1 receptor domain containing adaptor inducing interferon-β/TRIF, and TRIF-related adaptor molecule (TRAM) to activate transcription factors such as NF-κB and activator protein 1 (AP-1) and interferon regulatory factors (IRFs) that at the end lead to production of a multitude of inflammatory cytokines and initiation of innate immunity (64).
Activated HSC express TLR2, TLR3, TLR4, TLR7, and TLR9 and respond to respective ligands (LPS, Lipid A) with altered expression of IL-8, IL-6, Intercellular Adhesion Molecule 1 (ICAM-1), VCAM-1, TGF-β1, MCP-1/CCL2 and BAMBI (65-74). Therefore, all these TLRs have strong implications for disease progression and in regard to liver immunology it is assumed that the effective therapeutic targeting of TLRs in activated HSC could prevent the induction of many immune and inflammatory responses that are the hallmark in all liver diseases. In the following, we will therefore shortly discuss the significance of individual TLRs in HSC biology.
Toll-like receptor 2 (TLR2)
TLR2 (CD282) that responds to lipid-containing PAMPs is required for the activation of the NLRP3 inflammasome and contributes to liver inflammation and fibrosis (75). Mice that lack TLR2 are more susceptible to LPS-induced inflammatory responses or carbon tetrachloride (CCl4) -induced liver damage than wild type mice (76-78). In HSC, the TLR2 pathway also mediates biological effects of the hepatitis C virus core protein such as the induction of expression of profibrogenic marker proteins and signalling molecules (79).
Toll-like receptor 3 (TLR3)
TLR3 (CD283) recognizes double stranded RNA associated with viral infections. One of the consequences of TLR3 activation is the induction of NF-κB and the expression of IRF3 leading to production of type I IFN (80). The antiviral effects of TLR3 in human HSC comprise production of antiviral cytokines, induced expression of IRF7, upregulation of interferon-stimulated genes, and activation of retinoic acid-inducible gene-1 (RIG-1) expression resulting in significant suppression of Hepatitis C virus replication (70,81). Recently, it was shown that stimulation of TLR3 ameliorates alcoholic liver injury via stimulation of IL-10 production in HSC and KC, again demonstrating that TLR3 acts as an innate immune sensor triggering anti-inflammatory pathways in response to injury or infection (82). This assumption was recently impressively underpinned by Wilson and coworkers who showed that quiescent HSC isolated from tlr3 deficient mice do not produce IFN-γ in response to treatment with the TLR3 agonist polyI:polyC (74).
Toll-like receptor 4 (TLR4)
TLR4 (CD284) is the major receptor implicated in signal transduction events induced by LPS (83). In human HSC the activation of TLR4 by LPS or Lipid A results in activation of NF-κB and JNK pathways and increased expression of several chemokines and adhesion molecules (65). In line with this assumption, it was demonstrated that an artificial LPS and Lipid A sequestering soluble fusion construct made out of TLR4 and MD-2 was able to inhibit LPS-induced NF-κB and JNK activation (84). The activation of this receptor in quiescent HSC downregulates the TGF-β pseudo receptor BAMBI, thereby sensitizing these cells for TGF-β and providing a direct link between pro-inflammatory and profibrogenic signals (67). In humans, several single nucleotide polymorphisms are identified that predict reduced TLR4 responsiveness and confer a significantly reduced risk for fibrosis progression (85-87). TLR4 and its signalling pathways in liver injury are the most studied from all TLR family members, and there is a bulk of literature available that provides detailed information on individual components of TLR4 signalling components including receptors, adaptors, ligands, transcription factors, downstream factors and its implication in liver injury (88). Mechanistically, it was assumed that TLR4 signalling mediates an endotoxin-stimulated inflammatory phenotype of activated HSC that contributes to cell survival (89). Therefore, the response of HSC to endotoxins and TLR-associated signalling pathways is considered to have strong implications for hepatic inflammation and immune regulation (72).
Toll-like receptor 7 (TLR7)
TLR7 recognises single-stranded RNA and guanosine analogues in endosomes (89). Interestingly, HSC that were isolated from rats that underwent bile duct ligature for two weeks exhibited reduced expression of TLR7, while the treatment with the TLR7/8 agonist CL075, a thiazoloquinolone derivative, significantly reduced expression of CCL2 (MCP-1), TGF-β1, collagen type I, MMP2 as well as HSC proliferation and migration (90). This data indicate that TLR7-dependent pathways in HSC are effective in modulating liver injury, inflammation or fibrogenesis and support the idea that targeting TLR7 provides a promising new strategy for treatment of cholestatic liver diseases.
Toll-like receptor 9 (TLR9)
TLR9 (CD289) recognizes unmethylated CpG sequences in DNA that have a high frequency in bacteria. Accordingly, TLR9 deficient mice are resistant to CpG DNA-induced inflammatory T-helper type-1 responses and to proliferation of splenocytes (91). During activation of HSC, the expression of TLR9 is elevated and the stimulation of HSC with the synthetic TLR9 inhibitor ODN TTAGGG blocks the CpG-induced upregulation of TGF-β and collagen mRNA induced by apoptotic hepatocyte DNA (63). Similarly, the activation of TLR with the synthetic agonistic oligonucleotide ODN M362 inhibited PDGF signalling and PDGF-induced HSC chemotaxis (68). In contrast to these findings, it was demonstrated that the disruption of TLR9 in mice significantly attenuates ongoing fibrogenesis in experimental models of obstructive cholestasis, nonalcoholic steatohepatitis, and liver ischemia-reperfusion injury (69,71,92). Studies in which lymphocyte/HSC interactions were analysed revealed the complexity of TLR9 responses (93). However, all these findings provided evidence that the TLR9 pathway modulates the hepatic immunology and the outcome of liver diseases.
Other TLRs in HSC
Most of the TLRs are detectable in human and mouse HSC (81). However, the significance of TLR1, TLR5, TLR6, and TLR8 expression in HSC and its impact on liver immunology is presently not known. Based on their ligands that encompass multiple triacyl lipopeptides (TLR1, TLR6), bacterial globular proteins of the flagellum (TLR5), and small synthetic compounds as well as single stranded RNA (TLR8), it is tempting to speculate that the emerging field of TLR research will also find some roles for these TLRs in liver immunology and particularly in HSC.
IL-17, IL-22, IL-23, IL-27, IL-33, and IL-8
Most of the ILs are indispensable for proper function of the immune system. In fact, upregulation of manifold ILs is a hallmark feature of the inflamed microenvironment resulting from liver injury (94). From the group of approximately 40 different ILs, some ILs were recently linked to HSC activation and function.
Interleukin 17 (IL-17)
The six members of the IL-17 family (IL-17A-F) are primarily involved in mediating proinflammatory responses such as production of many other cytokines and chemokines. In the liver, IL-17 contributes to the pathogenesis of alcoholic hepatitis and is a key feature contributing to liver neutrophil recruitment (95). In the pathogenesis of liver fibrosis in mice, the expression of both IL-17 and its receptor IL-17RA increases and IL-17 directly induces production of collagen expression in HSC via the signal transducer and activator of transcription 3 (STAT3) pathways (96). Mice that are deficient for IL-17 show less liver damage after subjection to bile duct ligation (BDL). In a recent study, fibrotic livers taken from hepatitis B virus (HBV) -infected patients that underwent liver surgery (partial hepatectomy) had higher expression of IL-17A with highest immunoreactivity in regions with inflammatory infiltrates (97). The stimulation of cultured HSC with LPS results in a forty times elevated expression of IL-17F after 24 hr suggesting a unique regulatory potential of these hepatic cell population in hepatic immune responses (72). Of note, IL-17 is not only relevant for HSC in the context of liver inflammation, but certainly also a characteristic feature of T helper cell subsets (Th17 cells) and gamma-delta T cells (98,99).
Interleukin 22 (IL-22)
The significance of IL-22 and its receptors IL-10R2 and IL-22R1 in HSC biology was recently demonstrated (100). In HSC, IL-22 induces senescence through the activation of the STAT3 pathway, SOCS3, p53, and p21 thereby allowing to limit liver fibrosis and acceleration of resolution of liver fibrosis (100). Other reports have demonstrated that IL-22 treatment stimulates the secretion of several chemokines in HSC. In line, numerous IL-22 pathway-associated genes are significantly up-regulated in liver tissue of HBV-infected patients, most likely by IL-22 that is expressed by multiple intrahepatic immune cells and preferentially by T-helper 17 cells (101). These initial reports already suggest that IL-22 is another critical cytokine that contributes to the regulation of liver immunology.
Interleukin 23 (IL-23)
IL-23 is a heterodimer of the IL-6 family composed of two subunits (IL-12p40, IL-23p19) playing important roles in many immunological diseases. Since several years it is known that IL-23 has antitumor activity in murine models of hepatocellular carcinoma (102). Recently, it was demonstrated that IL-23 is specifically required for recruitment of neutrophils and TNF-α-/NO-producing monocytes to the liver, which are critical steps for bacterial clearance (103). The ubiquitous expression of the IL-23 subunit p19 resulted in constitutive expression of acute phase proteins in the liver and increased platelet production, while liver-specific expression of p19 does not result in a detectable phenotype (104). During HBV infection, it was shown that IL-23 is indispensable for HBV-antigen-stimulated IL-17 production. The IL-23-IL-17 axis is required to modulate the biological functionality of HSC and DCs that in turn can effectively polarize naïve CD4+ T cells towards the IL-17-producing Th17 phenotype (105).
Interleukin 27 (IL-27)
IL-27 is secreted by antigen-presenting cells and acts on T cells and other immune cells. It has recently attracted much attention due to its ability to exert IFN-γ-like functions in hepatocytes and its contribution to the antiviral response in these cells (106). It activates STAT1 and to a minor extent STAT3 in HSC leading to the induction of target genes such as interferon response factors 1 and 9, myxovirus resistance A, guanylate binding protein 2, and STAT1 itself (106). Likewise, IL-27 acts on and induces IFN-γ-like functions in HSC suggesting that this cytokine has a more general role in antiviral and inflammatory responses in the liver (107).
Interleukin 33 (IL-33)
IL-33 is a member of the IL-1 cytokine family that activates NF-κB and MAP kinases pathways and stimulates the expression of various cytokines in Th2-polarized naïve T cells (108). It is increasingly recognized by hepatologists because (I) its overexpression was found to be strongly associated with hepatic fibrosis in mice and humans; and (II) major sources of IL-33 in normal liver are HSC and sinusoidal endothelial cells (109). Mechanistically, the profibrogenic effects of IL-33 alone are sufficient to establish severe hepatic fibrosis that is primarily related to activation and expansion of liver resident innate lymphoid cells (110).
Interleukin 8 (IL-8)
LPS up-regulates IL-8 gene expression and secretion in a NF-κB-dependent pathway (65). Similarly, CD40 activation was shown to increase the secretion of IL-8 and monocyte chemoattractant protein-1 by HSC (111). However, in humans the activity of NF-κB and expression of IL-8 after induction with LPS shows a high variability, therefore possibly explaining differences in the outcome of fibrosis progression and the degree of inflammation during chronic liver disease (112). IL-8 is induced in cells transfected with the HCV core protein and identified as one of the major triggers driving expression of α-smooth muscle actin in the human HSC line LX-2 as well as in primary HSC (113). Vice versa, in co-culture HSC have the capacity to stimulate IL-8 expression in HCV-infected hepatocytes, thus indicating that IL-8 is one of the key mediators for the crosstalk between different cell populations during HCV infection (114).
B7-H1 pathway
The B7 homolog 1 also known as PD-L1 or CD274 is a transmembrane protein with immune-suppressive functions. Initial reports have shown that the activation of HSC is associated with a markedly enhanced expression of B7-H1 and that its suppression lowers the immunomodulatory activity of HSC on T cells (12). Its expression is stimulated by IFN-γ and mediates MEK/ERK-dependent immune-suppressive effects on T-cells suggesting that it is one control mediator possibly relevant for rejection of liver allografts (115,116). In line with this assumption, it was shown that B7-H1 expression on HSC can mediate profound T cell inhibitory activity in an islet allograft model in mice (117). Another previous report that highlighted the immune regulatory role of activated HSC in promotion of HCC growth concluded that the presence of activated HSC promotes tumor angiogenesis and HCC cell proliferation, in which high-level expression of B7-H1 in HSC might be one potential key mechanism for the immune regulatory function of these cells (118).
HSC and IFN-γ
One of the most studied cytokine in liver immunology is IFN-γ, because it is a potent activator of macrophages and has critical roles in innate and adaptive immunity against viral, bacterial and protozoal infection. With respect to HSC it is known that IFN-γ reduces HSC activation both in vitro and in vivo (119,120). In vitro, IFN-γ induces Smad7 protein expression in a STAT1-dependent manner and further reduces expression and activation of profibrogenic Smad2/Smad3 (121). Global protein expression profiling in HSC that were treated with IFN-γ further revealed that IFN-γ suppresses TGF-β- and PDGF-dependent signalling pathways, impacts the organisation of intermediate filaments, triggers fatty acid metabolism, and decreases expression of genes that control TNF-α activation (122). Moreover, IFN-γ induces apoptosis in activated HSC suggesting that part of the favourable effects of IFN-γ might be due to HSC clearance (123). Based on all these positive effects on HSC, it is not surprising that the transient overexpression of IFN-γ was proposed to inhibit the progression of hepatic fibrosis in vitro and in vivo (124). The beneficial effects of intact IFN-γ signalling in HSC for hepatic immune tolerance, prevention of liver transplant rejection, and control of T cell activity was also demonstrated in other experimental models (117).
ICAM-1 in HSC
The glycoprotein ICAM-1 (CD54) is a typical marker of endothelial cells and cells of the immune system; ICAM-1 binds integrins and has essential functions in inflammatory responses (125). ICAM-1 expression in HSC was first demonstrated in a differential polymerase chain reaction display technique that compared quiescent and activated HSC showing that ICAM-1 is significantly upregulated during prolonged culturing and that its expression is elevated in rat livers that were experimentally injured by BDL (126). While the authors of that study suggested that ICAM-1 expression in activated HSC is mainly implicated in the transmigration of leukocytes from the hepatic sinusoid to sites of liver damage during the inflammatory response in the liver, other studies suggested that ICAM-1 expression is necessary for proper HSC immune inhibitory activities both in vitro and in vivo (127). Later studies have argued that increased expression of ICAM-1 on HSC restricts the expression of IL-2 and IL-2 receptor expression in T cells that in consequence leads to an attenuation of T cell activation by DCs.
Mechanistic aspects of HSC on liver immunology
Antigen presentation
In adaptive immunity, the process of antigen presentation is one critical process that is necessary to allow antigen-specific priming of T cells and to allow them to recognize a particular antigen as “non-self”. Professional APCs are DCs, but also macrophages, monocytes and B lymphocytes. These cells internalize foreign antigens by phagocytosis or receptor-mediated endocytosis and display parts of them at their surface via class II major histocompatibility complex (MHC) molecules. Already in 2003, it was reported that cultured HSC also display features of APC (128). In this pioneering study it was demonstrated that human HSC express a large variety of membrane receptors including members of the HLA family, lipid-presenting molecules, factors that are directly involved in T cell activation and proteins that are involved in antigen trafficking (128). Interestingly, this study has also shown that the exposure of HSC to proinflammatory stimuli results in a significant upregulation of respective genes suggesting that the APC function is another attribute of activated HSC in the context of liver inflammation. A subsequent study further revealed the expression of receptors for the Fc fragment of IgG by rat HSC (129). Rat HSC also express the HLA class II histocompatibility antigen-γ chain (CD74) and cathepsin S that is an essential lysosomal cysteine protease participating in the degradation of antigenic proteins (130).
In functional studies, it was demonstrated that murine HSC can efficiently present antigens to CD1-, MHC-I-, and MHC-II-restricted T cells and to elicit a multitude of T cell responses specific for protein and lipid antigens (131). Moreover, the study provided evidence that transgenic HSC can present their endogenous peptide neo-antigens to peptide-specific T cells in vivo. In line with this assumption, it was later shown that also rat HSC are able to process and present exogenous antigens and that the APC function is markedly decreased in fully transdifferentiated MFB (132). Another report has suggested that HSC alone do not present antigens and require the presence of retinoic acid, DCs and TGF-β1 to induce functional (mostly regulatory) T cells (44). The repertoire of HSC activity in antigen presentation was further extended by the finding that HSC can possess a transdifferentiation-dependent veto function, in which they prevent the activation of naïve T cells by DCs in a cell contact-dependent fashion (133). In addition, conditioned culture supernatant from activated human HSC have the capacity to significantly promote the differentiation of human peripheral blood mononuclear cells to macrophages demonstrating that HSC secrete signalling molecules that impacts the function or differentiation of immune cells (134). All these studies show that HSC represent liver-resident professional APC that either can stimulate or repress specific immunological processes in the liver.
HSC and inflammasome
The inflammasomes are cytoplasmic multiprotein complexes that are assembled in immune cells during phases of cellular injury and death. Likewise, cellular stress, viral or bacterial infection, free cytoplasmic DNA, or any other kind of injury that leads to the activation of specialized receptors results in the formation of these high-molecular-mass inflammasome platforms. Once activated, they trigger the activation of inflammatory caspases and the maturation of pro-inflammatory cytokines such as IL-1β (135,136). Specialized intracellular sensors, i.e., the NOD-like receptors (NLRs) that recognize pathogen-associated molecular patterns are a major part of these platforms. We have recently shown that several of these receptors (NLRP1,NLRP3, AIM2) are expressed in KC, liver sinusoidal endothelial cells, in periportal MFB and also in HSC, while the expression of these receptors in hepatocytes were only detectable when they were exposed to appropriate inflammatory stimuli (137). Challenge with LPS drastically increases expression of the NLRs in cultured HSC suggesting that the activation of inflammasomes is one additional facet that allows HSC to contribute to liver immunology (137). A previous study has also documented that the application of monosodium urate crystals into cultures of HSC results in a strong upregulation of TGF-β1 and collagen type 1 mRNA expression and actin reorganization that was not observed when individual inflammasome components were lacking (138). Presently, it is not known if the activation of the different inflammasome branches in HSC requires TLR signalling, since a recent study demonstrated that TLR2 ligands alone are not sufficient to induce inflammasome activity in HSC (75).
HSC and autophagy
The term “autophagy” was first introduced in the literature by de Duve in 1963 and describes the phenomenon of cell degradation and recycling of damaged own cellular organelles and large protein aggregates through lysosomes (139). In this process, the intracellular degradation takes place in an intracellular degradation system, the autophagosome that is a spherical structure surrounded by double layer membranes (140). This process is also essentially involved in minimizing ROS formation, hence quenching the activation of intracellular pro-inflammatory factors and pathways that trigger intracellular of inflammasome activation (141). In liver, there is clear evidence that autophagy activity is important for the balance of energy and nutrients during cell homeostasis and that the autophagy capacity decreases during aging (142). Upon activation of murine and human HSC the process of autophagy is significantly increased (143). Also in vivo the autophagic activity in HSC increases during experimental liver injury, while the loss of autophagy in cultured HSC and in injured livers reduced fibrogenesis confirming that autophagic activity is required to establish an activated phenotype in HSC (144). Since liver fibrosis may ultimately progress to cirrhosis and liver cancer, these data suggests that autophagy is another mechanism that might influence immune surveillance in the liver.
Apoptotic body engulfment
The clearance of apoptotic cell corpses termed “apoptotic body engulfment” is the last step of programmed cell death. It is a highly orchestrated process in which “find me” and “eat me” signals are just as important as regulatory mechanisms that drive cytoskeletal rearrangement for engulfment and signals rendering the phagocytic cell immunologically silent after successful engulfment (145). HSC can phagocytose hepatocyte-derived apoptotic bodies, which results in the activation of HSC (146). The uptake of apoptotic bodies by HSC increased ROS formation, NADPH oxidase activation, and further induces different JAK/STAT and Akt/NF-κB-dependent survival pathways in HSC (147,148). Since the clearance of apoptotic bodies is one essential mechanism for the attenuation of inflammatory responses as well as for initiating signals for tissue repair and remodelling in response to cell death (149), it is obvious that also engulfment of apoptotic bodies is one addition process relevant for liver immunity.
HSC in hepatocellular carcinoma and enhancement of immunosuppressive cells
There is increasing evidence that the immunomodulatory activities of HSC can promote the occurrence, development and progression of hepatocellular carcinoma or other malignancies in vitro and in vivo (150,151). In a xenograft model, in which human colorectal tumor cells were grown in the liver, there was strong evidence that HSC were overrepresented at the invasion front and that activated HSC are the predominant cell type that is involved in the extensive cellular communication that is necessary to remodel the extracellular matrix and the host reaction on metastatic invasion in the tumor area (152). One potential mechanism that is presently discussed is that activated HSC promote T cell apoptosis and change the balance of different T cell populations, thereby enhancing immunosuppressive cell populations in the HCC microenvironment (153,154). In another concept, malignant hepatocytes and activated HSC are thought to form together with cancer-associated fibroblasts and immune cells a tumour-associated milieu in which the responsiveness of tumour cells towards soluble factors is enhanced (155). Therefore, activated HSC are major players in the formation of the tumour cell microenvironment that decides about the malignancy and outcome of the tumour event.
Cell-cell-interactions of HSC with immune cells
Due to their anatomical position in the space of Dissé, between the fenestrated endothelium of the sinusoids and the hepatocytes, HSC are optimally located to interact with many cell types in the liver. For instance, HSC closely interact with sinusoidal endothelial cells, as HSC can provide angiogenic factors like VEGF or angiopoietin-1, while they respond to PDGF, TGF-β, NO or other angiocrine factors released from the endothelium (156,157). The close contact to different cell types within the liver but also to bypassing circulating leukocytes gives HSC a central position and allows establishing a diversity of autocrine and paracrine regulations. In this network of cellular interactions, HSC endorse decisive signals to immune cells and vice versa receive signals that modulate the biological properties of HSC itself (Figure 3). Some of these important cell-cell interactions will be discussed in the following sections.
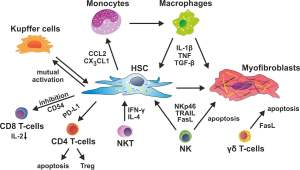
Macrophages
As pointed out above, HSC express manifold receptors and soluble effector molecules that enable them to interact with distinct immune cell subsets. Among these, the interaction between HSC and macrophages is of outstanding importance for the pathogenesis of chronic liver diseases. This is already evident from in vitro studies using gene array and proteomic profiling of HSC, because considerable differences between in vitro and in vivo activated HSC were noted (158,159). The isolation of HSC from livers of mice that had been subjected to either CCl4 or BDL induced experimental fibrosis showed highly correlated gene expression patterns for these in vivo activated HSC, including pro-inflammatory and anti-apoptotic mediators, transcription factors, cell surface receptors and cytoskeleton components, similar to human HSC from cirrhotic livers (158,160). While culture activation by itself only partially reproduced these characteristic changes, the co-culture of HSC with KC more closely resembled the in vivo situation (158), indicating the relevance of macrophage-HSC interactions in vivo.
Liver fibrosis models in mice provided strong experimental evidence for the close interaction between inflammatory signals, monocytes/macrophages and HSC driving fibrogenesis in vivo. HSC as well as KC, the resident macrophage population in the liver, respond to PAMP or DAMP via TLR4 (67,161). Activated HSC then release an array of pro-inflammatory chemokines [CCL2, CCL4, fractalkine (CX3CL1) and others] (67,161). These chemokines attract monocytes, mainly the inflammatory Ly6C (Gr1) expressing subset, from the peripheral blood into the injured liver (162). Inflammatory monocyte-derived macrophages produce TGF-β which further stimulates HSC activation (163). Moreover, hepatic macrophages release several pro-inflammatory cytokines like TNF or IL-1 (164), which activate the transcription factor NF-κB in HSC and thereby promote the survival of activated HSC and MFB (165).
In humans, ‘non-classical’ CD14+ and CD16+ monocytes/macrophages appear to exert similar pro-inflammatory and pro-fibrogenic actions on HSC. When different monocyte subsets are isolated from patients with hepatic fibrosis, the CD14+CD16+ monocyte subset release many inflammatory cytokines and can efficiently activate primary HSC in vitro (166,167). Interestingly, not only monocytes and macrophages influence HSC differentiation and survival, but HSC also impact on the macrophage phenotype. When human HSC were co-cultured with human peripheral blood monocytes, only activated but not freshly isolated HSC were capable of inducing a specific CD14+HLA-DR-/low phenotype in the co-cultured monocytes. These monocyte-derived cells suppressed T-cell proliferation in an arginase-1 dependent fashion, thereby resembling the phenotype of “monocyte-derived suppressor cells” (MDSC) known from tumor microenvironment. Importantly, MDSC-induction by HSC was at least partly mediated by CD44-dependent cell-cell-contacts (168). It is intriguing to speculate that the local induction of MDSC by activated HSC could contribute to immune suppression in inflamed liver.
Dendritic cells (DC)
DCs are a rather heterogeneous population in the liver and include monocyte-/macrophage-derived cells with DC functions, plasmacytoid DC and populations of conventional DC (169). Similar to hepatic macrophages, DCs do not activate HSC in co-culture systems, but up-regulate expression of NF-κB dependent genes through an IL-1 and TNF-dependent mechanism (165). However, this induction is much lower compared to effects on HSC exerted by macrophages, and the DC-HSC interaction was found to be irrelevant for the overall phenotype of experimental fibrosis models in vivo (165).
NK and NKT cells
Liver lymphocytes are highly enriched with NK as well as NKT cells. While NK cells are important for killing virally infected or transformed hepatocytes (170), NKT cells patrol the liver and are capable of rapidly aggravating inflammatory responses in conditions of injury (171). Experimental evidence indicates that both cell types impact the functionality of HSC in liver fibrosis. For instance, mouse models of hepatic fibrosis revealed that NK cells, via their activating receptor NKp46, selectively kill early or senescence activated HSC (170,172). Similarly, human NK cells from HCV-infected patients were shown to induce apoptosis in activated primary human HSC, in a TRAIL-, FasL- and NKG2D-dependent manner (173). This NK cell-mediated cytotoxicity on HSC was related to the stage of fibrosis in patients as well as to the presence of activating (e.g., IFN-α) or inhibiting (e.g., ethanol, TGF-β) factors in vitro (170,174). Moreover, NK cells can produce IFN-γ, which has additional anti-fibrotic properties (175).
NKT cells, an unconventional T cell population expressing both NK and T cell markers, were found to promote liver fibrogenesis in vivo, likely by enhancing inflammatory responses via releasing pro-inflammatory cytokines and by activating HSC via osteopontin and Hh ligands (171,176). However, there are also studies demonstrating that NKT cells may exert antifibrotic actions, because they can, under certain conditions, also kill HSC and produce IFN-γ (170,177). Interestingly, another type of unconventional T cells, the gamma delta T cell receptor expressing T cells (γδ T cells), have the ability to induce HSC apoptosis in a cell-cell contact-dependent manner, via expression of Fas-ligand (CD95L) on their surface (99).
T cells
While the direct antigen presentation capacity of HSC is still controversial (see above), there is clear evidence that HSC can interact with T lymphocytes and suppress adaptive immune responses. Mouse as well as human activated HSC express B7-H1 (PDL-1), which inhibits T cell responses by inducing T cell apoptosis via B7-H1/PD-1 ligation in vitro and in vivo (12,13,116). Furthermore, activated HSC express the coinhibitory molecule B7-H4, which inhibits early T cell activation and results in T cell anergy (178). There is also evidence that HSC might preferentially induce immune-suppressive Treg in an IL2-dependent fashion (179,180).
In addition, HSC might also restrict cytotoxic responses by CD8 T cells. HSC were found to inhibit the activation of naïve CD8 T cells by expressing high levels of CD54 and confining the expression of IL-2 and IL-2R on T cells. This particular HSC activity was termed “veto function” and correlated with the degree of HSC activation, being most pronounced in HSC from fibrotic livers (125).
HSC and immune cell crosstalk in the resolution of fibrosis
Intriguing data from patients infected with HBV and undergoing long-term effective antiviral therapy provided clinical evidence that fibrosis is not a unidirectional process of progressive disease, but can actually resolve and revert to normal tissue (181). Over the past decades, the highly dynamic nature of tissue repair and remodelling in liver fibrosis resolution has become apparent (182). Important mechanisms for fibrosis regression are MFB apoptosis, matrix degradation by metalloproteinases and the central involvement of hepatic macrophages in orchestrating this process (183). With respect to macrophages, a subset of “restorative macrophages” has been identified in mouse models of fibrosis regression, which originates from infiltrating monocytes, expresses high levels of matrix metalloproteinases and is likely induced by phagocytosis of apoptotic material (184,185). Up to now, most studies focussed on these immune mechanisms during tissue repair and noticed that MFB, which are derived from activated HSC, undergo apoptosis. More recently, HSC “deactivation” was identified as an important mechanism during fibrosis resolution. Using sophisticated cell tracking methods in mice, about half of the MFB escape apoptosis during regression of liver fibrosis, down-regulate fibrogenic genes, and acquire a phenotype similar to quiescent HSC (186). However, these “reverted” (or deactivated) HSC remain in a primed state, meaning that they easily reactivate into MFB in response to recurring fibrogenic stimuli (186,187). The molecular signals and cell-cell-dependent contacts mediating HSC deactivation are currently largely obscure. It is very likely that, besides NK/NKT cell-HSC interactions that promote HSC apoptosis, interactions between HSC and distinct immune cell subpopulations, likely restorative macrophages, promote HSC deactivation. Further research is eagerly warranted as novel therapeutic concepts could aim at augmenting HSC deactivation.
Concluding remarks
Experimental models in mice and rats as well as analyses from human liver unravelled a central role for HSC in maintaining homeostasis in the liver as well as critical functions for the pathogenesis of liver diseases. While most studies in the past focussed on the crucial involvement of HSC in liver fibrogenesis as the main extracellular matrix producing cell population in fibrotic livers, more recent evidence revealed that HSC exert important tasks in regulating inflammatory as well as immunological processes in injured liver. Importantly, it is increasingly recognized that HSC represent a phenotypically and likely functionally heterogeneous cell population. The state of HSC activation as well as the nature of the underlying liver injury (e.g., acute vs. chronic) further contributes to immune-regulatory functions of HSC. The number of HSC-elaborated inflammatory and immune regulatory molecules that contribute to liver immunology may be even much greater than known today. However, it became apparent that HSC are rather immune-suppressive in homeostasis, but are important sensors of altered tissue integrity and initiators of innate immune cell activation. Vice versa, several immune cell subtypes interact directly or via soluble mediators with HSC in health and disease. The tremendous progress in understanding the role of HSC as central regulators of liver immunology gives rise to the expectation that targeting these pathways may lead to novel therapeutic strategies for chronic liver diseases.
Acknowledgements
The authors thank all members of the Weiskirchen and Tacke labs for helpful discussions. We are grateful to Sabine Weiskirchen for assistance with the figure design. This work was supported by the German Research Foundation (DFG SFB/TRR57).
Disclosure: The authors declare no conflict of interest.
References
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-β as major players and therapeutic targets. J Cell Mol Med 2006;10:76-99. [PubMed]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-72. [PubMed]
- Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:2823. [PubMed]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 2001;21:311-35. [PubMed]
- Wake K, Sato T. Intralobular heterogeneity of perisinusoidal stellate cells in porcine liver. Cell Tissue Res 1993;273:227-37. [PubMed]
- Higashi N, Senoo H. Distribution of vitamin A-storing lipid droplets in hepatic stellate cells in liver lobules--a comparative study. Anat Rec A Discov Mol Cell Evol Biol 2003;271:240-8. [PubMed]
- Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol 2012;6:67-80. [PubMed]
- Maxwell PH, Ferguson DJ, Osmond MK, et al. Expression of a homologously recombined erythopoietin-SV40 T antigen fusion gene in mouse liver: evidence for erythropoietin production by Ito cells. Blood 1994;84:1823-30. [PubMed]
- Schmeding M, Neumann UP, Boas-Knoop S, et al. Erythropoietin reduces ischemia-reperfusion injury in the rat liver. Eur Surg Res. 2007;39:189-97. [PubMed]
- Schmeding M, Rademacher S, Boas-Knoop S, et al. rHuEPo reduces ischemia-reperfusion injury and improves survival after transplantation of fatty livers in rats. Transplantation 2010;89:161-8. [PubMed]
- Park SY, Lee JY, Tak WY, et al. Erythropoietin decreases carbon tetrachloride-induced hepatic fibrosis by inhibiting transforming growth factor-beta. Chin Med J (Engl) 2012;125:3098-103. [PubMed]
- Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004;40:1312-21. [PubMed]
- Chen CH, Kuo LM, Chang Y, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology 2006;44:1171-81. [PubMed]
- Maher JJ. Interactions between hepatic stellate cells and the immune system. Semin Liver Dis 2001;21:417-26. [PubMed]
- Dranoff JA, Ogawa M, Kruglov EA, et al. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2004;287:G417-24. [PubMed]
- Wasmuth HE, Weiskirchen R. Pathogenesis of liver fibrosis: modulation of stellate cells by chemokines. Z Gastroenterol 2010;48:38-45. [PubMed]
- Puche JE, Lee YA, Jiao J, et al. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology 2013;57:339-50. [PubMed]
- McLaren DS, Kraemer K. Retinoids and carotenoids in general medicine. World Rev Nutr Diet 2012;103:137-47. [PubMed]
- Natarajan SK, Thomas S, Ramachandran A, et al. Retinoid metabolism during development of liver cirrhosis. Arch Biochem Biophys 2005;443:93-100. [PubMed]
- Aguilar RP, Genta S, Oliveros L, et al. Vitamin A deficiency injures liver parenchyma and alters the expression of hepatic extracellular matrix. J Appl Toxicol 2009;29:214-22. [PubMed]
- Nollevaux MC, Guiot Y, Horsmans Y, et al. Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption. Liver Int 2006;26:182-6. [PubMed]
- Nakane PK. Ito’s fat storing cell of the mouse liver. Anat Rec 1963;145:265-6.
- Wake K. “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat 1971;132:429-62. [PubMed]
- Hendriks HF, Verhoofstad WA, Brouwer A, et al. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res 1985;160:138-49. [PubMed]
- Kluwe J, Wongsiriroj N, Troeger JS, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut 2011;60:1260-8. [PubMed]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167-92. [PubMed]
- Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res 2004;297:68-81. [PubMed]
- Dawson HD, Li NQ, DeCicco KL, et al. Chronic marginal vitamin A status reduces natural killer cell number and function in aging Lewis rats. J Nutr 1999;129:1510-7. [PubMed]
- Twining SS, Schulte DP, Wilson PM, et al. Vitamin A deficiency alters rat neutrophil function. J Nutr 1997;127:558-65. [PubMed]
- Ahmad SM, Haskell MJ, Raqib R, et al. Markers of innate immune function are associated with vitamin a stores in men. J Nutr 2009;139:377-85. [PubMed]
- Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm 2011;86:103-26. [PubMed]
- Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology 2011;53:1007-19. [PubMed]
- Zhang Y, Chen Q, Ross AC. Retinoic acid and tumor necrosis factor-α induced monocytic cell gene expression is regulated in part by induction of transcription factor MafB. Exp Cell Res 2012;318:2407-16. [PubMed]
- Beijer MR, Kraal G, den Haan JM. Vitamin A and dendritic cell differentiation. Immunology 2014;142:39-45. [PubMed]
- Chen Q, Mosovsky KL, Ross AC. Retinoic acid and α-galactosylceramide regulate the expression of costimulatory receptors and transcription factors responsible for B cell activation and differentiation. Immunobiology 2013;218:1477-87. [PubMed]
- van de Pavert SA, Ferreira M, Domingues RG, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014;508:123-7. [PubMed]
- Hoglen NC, Abril EA, Sauer JM, et al. Modulation of Kupffer cell and peripheral blood monocyte activity by in vivo treatment of rats with all-trans-retinol. Liver 1997;17:157-65. [PubMed]
- Mehta K, McQueen T, Tucker S, et al. Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J Leukoc Biol 1994;55:336-42. [PubMed]
- Mathew JS, Sharma RP. Effect of all-trans-retinoic acid on cytokine production in a murine macrophage cell line. Int J Immunopharmacol 2000;22:693-706. [PubMed]
- Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ 1993;306:366-70. [PubMed]
- Ahmad SM, Haskell MJ, Raqib R, et al. Men with low vitamin A stores respond adequately to primary yellow fever and secondary tetanus toxoid vaccination. J Nutr 2008;138:2276-83. [PubMed]
- Lee KA, Song YC, Kim GY, et al. Retinoic acid alleviates Con A-induced hepatitis and differentially regulates effector production in NKT cells. Eur J Immunol 2012;42:1685-94. [PubMed]
- Ichikawa S, Mucida D, Tyznik AJ, et al. Hepatic stellate cells function as regulatory bystanders. J Immunol 2011;186:5549-55. [PubMed]
- Dunham RM, Thapa M, Velazquez VM, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol 2013;190:2009-16. [PubMed]
- Cravedi P, van der Touw W, Heeger PS. Complement regulation of T-cell alloimmunity. Semin Nephrol 2013;33:565-74. [PubMed]
- Schieferdecker HL, Rothermel E, Timmermann A, et al. Anaphylatoxin C5a receptor mRNA is strongly expressed in Kupffer and stellate cells and weakly in sinusoidal endothelial cells but not in hepatocytes of normal rat liver. FEBS Lett 1997;406:305-9. [PubMed]
- Schlaf G, Schieferdecker HL, Rothermel E, et al. Differential expression of the C5a receptor on the main cell types of rat liver as demonstrated with a novel monoclonal antibody and by C5a anaphylatoxin-induced Ca2+ release. Lab Invest 1999;79:1287-97. [PubMed]
- Hillebrandt S, Wasmuth HE, Weiskirchen R, et al. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet 2005;37:835-43. [PubMed]
- Xu R, Lin F, He J, et al. Complement 5a stimulates hepatic stellate cells in vitro, and is increased in the plasma of patients with chronic hepatitis B. Immunology 2013;138:228-34. [PubMed]
- Das D, Barnes MA, Nagy LE. Anaphylatoxin C5a modulates hepatic stellate cell migration. Fibrogenesis Tissue Repair 2014;7:9. [PubMed]
- Baumann M, Witzke O, Canbay A, et al. Serum C3 complement concentrations correlate with liver function in patients with liver cirrhosis. Hepatogastroenterology 2004;51:1451-3. [PubMed]
- Knittel T, Fellmer P, Neubauer K, et al. The complement-activating protease P100 is expressed by hepatocytes and is induced by IL-6 in vitro and during the acute phase reaction in vivo. Lab Invest 1997;77:221-30. [PubMed]
- Fimmel CJ, Brown KE, O'Neill R, et al. Complement C4 protein expression by rat hepatic stellate cells. J Immunol 1996;157:2601-9. [PubMed]
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet 2011;12:393-406. [PubMed]
- Omenetti A, Choi S, Michelotti G, et al. Hedgehog signaling in the liver. J Hepatol 2011;54:366-73. [PubMed]
- Sicklick JK, Li YX, Choi SS, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest 2005;85:1368-80. [PubMed]
- Lin N, Tang Z, Deng M, et al. Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2008;372:260-5. [PubMed]
- Kim Y, Kim MO, Shin JS, et al. Hedgehog signaling between cancer cells and hepatic stellate cells in promoting cholangiocarcinoma. Ann Surg Oncol 2014;21:2684-98. [PubMed]
- Yang IV, Alper S, Lackford B, et al. Novel regulators of the systemic response to lipopolysaccharide. Am J Respir Cell Mol Biol 2011;45:393-402. [PubMed]
- Furmanski AL, Saldana JI, Ono M, et al. Tissue-derived hedgehog proteins modulate Th differentiation and disease. J Immunol 2013;190:2641-9. [PubMed]
- Nakamoto N, Kanai T. Role of toll-like receptors in immune activation and tolerance in the liver. Front Immunol 2014;5:221. [PubMed]
- Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 1999;189:1777-82. [PubMed]
- Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem 2001;268:4580-9. [PubMed]
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 2008;48:322-35. [PubMed]
- Paik YH, Schwabe RF, Bataller R, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37:1043-55. [PubMed]
- Paik YH, Lee KS, Lee HJ, et al. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest 2006;86:676-86. [PubMed]
- Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007;13:1324-32. [PubMed]
- Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 2007;46:1509-18. [PubMed]
- Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 2010;139:323-34.e7.
- Wang Y, Li J, Wang X, et al. Induction of interferon-λ contributes to Toll-like receptor-3-activated hepatic stellate cell-mediated hepatitis C virus inhibition in hepatocytes. J Viral Hepat 2013;20:385-94. [PubMed]
- Gäbele E, Mühlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun 2008;376:271-6. [PubMed]
- Harvey SA, Dangi A, Tandon A, et al. The transcriptomic response of rat hepatic stellate cells to endotoxin: implications for hepatic inflammation and immune regulation. PLoS One 2013;8:e82159. [PubMed]
- Liu C, Chen X, Yang L, et al. Transcriptional repression of the transforming growth factor β (TGF-β) Pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by Nuclear Factor κB (NF-κB) p50 enhances TGF-β signaling in hepatic stellate cells. J Biol Chem 2014;289:7082-91. [PubMed]
- Wilson CL, Mann J, Walsh M, et al. Quiescent hepatic stellate cells functionally contribute to the hepatic innate immune response via TLR3. PLoS One 2014;9:e83391. [PubMed]
- Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2013;57:577-89. [PubMed]
- Szabo G, Velayudham A, Romics L Jr, et al. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res 2005;29:140S-145S. [PubMed]
- Rivera CA, Gaskin L, Allman M, et al. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol 2010;10:52. [PubMed]
- Ji L, Xue R, Tang W, et al. Toll like receptor 2 knock-out attenuates carbon tetrachloride (CCl4)-induced liver fibrosis by downregulating MAPK and NF-κB signaling pathways. FEBS Lett 2014;588:2095-100. [PubMed]
- Coenen M, Nischalke HD, Krämer B, et al. Hepatitis C virus core protein induces fibrogenic actions of hepatic stellate cells via toll-like receptor 2. Lab Invest 2011;91:1375-82. [PubMed]
- Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732-8. [PubMed]
- Wang B, Trippler M, Pei R, et al. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol 2009;51:1037-45. [PubMed]
- Byun JS, Suh YG, Yi HS, et al. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol 2013;58:342-9. [PubMed]
- Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest 2000;105:497-504. [PubMed]
- Schnabl B, Brandl K, Fink M, et al. A TLR4/MD2 fusion protein inhibits LPS-induced pro-inflammatory signaling in hepatic stellate cells. Biochem Biophys Res Commun 2008;375:210-4. [PubMed]
- Li Y, Chang M, Abar O, et al. Multiple variants in toll-like receptor 4 gene modulate risk of liver fibrosis in Caucasians with chronic hepatitis C infection. J Hepatol 2009;51:750-7. [PubMed]
- Von Hahn T, Halangk J, Witt H, et al. Relevance of endotoxin receptor CD14 and TLR4 gene variants in chronic liver disease. Scand J Gastroenterol 2008;43:584-92. [PubMed]
- Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 2009;49:960-8. [PubMed]
- Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010;3:21. [PubMed]
- Lee J, Chuang TH, Redecke V, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A 2003;100:6646-51. [PubMed]
- Chou MH, Huang YH, Lin TM, et al. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. Biochem J 2012;447:25-34. [PubMed]
- Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740-5. [PubMed]
- Bamboat ZM, Balachandran VP, Ocuin LM, et al. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology 2010;51:621-32. [PubMed]
- Abu-Tair L, Axelrod JH, Doron S, et al. Natural killer cell-dependent anti-fibrotic pathway in liver injury via Toll-like receptor-9. PLoS One 2013;8:e82571. [PubMed]
- Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol 2009;36:4-12. [PubMed]
- Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 2009;49:646-57. [PubMed]
- Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012;143:765-76.e1-3.
- Tan Z, Qian X, Jiang R, et al. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol 2013;191:1835-44. [PubMed]
- Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol 2011;2011:345803.
- Hammerich L, Bangen JM, Govaere O, et al. Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 2014;59:630-42. [PubMed]
- Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56:1150-9. [PubMed]
- Zhao J, Zhang Z, Luan Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014;59:1331-42. [PubMed]
- Hu P, Hu HD, Chen M, et al. Expression of interleukins-23 and 27 leads to successful gene therapy of hepatocellular carcinoma. Mol Immunol 2009;46:1654-62. [PubMed]
- Indramohan M, Sieve AN, Break TJ, et al. Inflammatory monocyte recruitment is regulated by interleukin-23 during systemic bacterial infection. Infect Immun 2012;80:4099-105. [PubMed]
- Wiekowski MT, Leach MW, Evans EW, et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J Immunol 2001;166:7563-70. [PubMed]
- Wang Q, Zhou J, Zhang B, et al. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog 2013;9:e1003410. [PubMed]
- Bender H, Wiesinger MY, Nordhoff C, et al. Interleukin-27 displays interferon-gamma-like functions in human hepatoma cells and hepatocytes. Hepatology 2009;50:585-91. [PubMed]
- Schoenherr C, Weiskirchen R, Haan S. Interleukin-27 acts on hepatic stellate cells and induces signal transducer and activator of transcription 1-dependent responses. Cell Commun Signal 2010;8:19. [PubMed]
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479-90. [PubMed]
- Marvie P, Lisbonne M, L’helgoualc’h A, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med 2010;14:1726-39. [PubMed]
- McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013;39:357-71. [PubMed]
- Schwabe RF, Schnabl B, Kweon YO, et al. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol 2001;166:6812-9. [PubMed]
- Mühlbauer M, Weiss TS, Thasler WE, et al. LPS-mediated NFkappaB activation varies between activated human hepatic stellate cells from different donors. Biochem Biophys Res Commun 2004;325:191-7. [PubMed]
- Clément S, Pascarella S, Conzelmann S, et al. The hepatitis C virus core protein indirectly induces alpha-smooth muscle actin expression in hepatic stellate cells via interleukin-8. J Hepatol 2010;52:635-43. [PubMed]
- Nishitsuji H, Funami K, Shimizu Y, et al. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol 2013;87:8169-78. [PubMed]
- Gu X, Wang Y, Xiang J, et al. Interferon- γ triggers hepatic stellate cell-mediated immune regulation through MEK/ERK signaling pathway. Clin Dev Immunol 2013;2013:389807.
- Charles R, Chou HS, Wang L, et al. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation 2013;96:17-24. [PubMed]
- Yang HR, Chou HS, Gu X, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology 2009;50:1981-91. [PubMed]
- Zhao W, Zhang L, Yin Z, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer 2011;129:2651-61. [PubMed]
- Rockey DC, Maher JJ, Jarnagin WR, et al. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology 1992;16:776-84. [PubMed]
- Baroni GS, D'Ambrosio L, Curto P, et al. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology 1996;23:1189-99. [PubMed]
- Weng H, Mertens PR, Gressner AM, et al. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol 2007;46:295-303. [PubMed]
- Fujita T, Maesawa C, Oikawa K, et al. Interferon-gamma down-regulates expression of tumor necrosis factor-alpha converting enzyme/a disintegrin and metalloproteinase 17 in activated hepatic stellate cells of rats. Int J Mol Med 2006;17:605-16. [PubMed]
- Saile B, Eisenbach C, Dudas J, et al. Interferon-gamma acts proapoptotic on hepatic stellate cells (HSC) and abrogates the antiapoptotic effect of interferon-alpha by an HSP70-dependant pathway. Eur J Cell Biol 2004;83:469-76. [PubMed]
- Chen M, Wang GJ, Diao Y, et al. Adeno-associated virus mediated interferon-gamma inhibits the progression of hepatic fibrosis in vitro and in vivo. World J Gastroenterol 2005;11:4045-51. [PubMed]
- Sligh JE Jr, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A 1993;90:8529-33. [PubMed]
- Hellerbrand, Wang SC, Tsukamoto H, et al. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology 1996;24:670-6.
- Yin Z, Jiang G, Fung JJ, et al. ICAM-1 expressed on hepatic stellate cells plays an important role in immune regulation. Microsurgery 2007;27:328-32. [PubMed]
- Viñas O, Bataller R, Sancho-Bru P, et al. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology 2003;38:919-29. [PubMed]
- Shen H, Zhang M, Kaita K, et al. Expression of Fc fragment receptors of immunoglobulin G (Fc gammaRs) in rat hepatic stellate cells. Dig Dis Sci 2005;50:181-7. [PubMed]
- Maubach G, Lim MC, Kumar S, et al. Expression and upregulation of cathepsin S and other early molecules required for antigen presentation in activated hepatic stellate cells upon IFN-gamma treatment. Biochim Biophys Acta 2007;1773:219-31.
- Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 2007;26:117-29. [PubMed]
- Bomble M, Tacke F, Rink L, et al. Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts. Biochem Biophys Res Commun 2010;396:342-7. [PubMed]
- Schildberg FA, Wojtalla A, Siegmund SV, et al. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology 2011;54:262-72. [PubMed]
- Chang J, Hisamatsu T, Shimamura K, et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatol Res 2013;43:658-69. [PubMed]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417-26. [PubMed]
- Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821-32. [PubMed]
- Boaru SG, Borkham-Kamphorst E, Tihaa L, et al. Expression analysis of inflammasomes in experimental models of inflammatory and fibrotic liver disease. J Inflamm (Lond) 2012;9:49. [PubMed]
- Watanabe A, Sohail MA, Gomes DA, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2009;296:G1248-57. [PubMed]
- Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy 2008;4:740-3. [PubMed]
- Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct 2002;27:421-9. [PubMed]
- Zhou R, Yazdi AS, Menu P, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221-5. [PubMed]
- Uddin MN, Nishio N, Ito S, et al. Autophagic activity in thymus and liver during aging. Age (Dordr) 2012;34:75-85. [PubMed]
- Thoen LF, Guimarães EL, Dollé L, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353-60. [PubMed]
- Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012;142:938-46. [PubMed]
- Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 2013;5:a008748. [PubMed]
- Canbay A, Taimr P, Torok N, et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest 2003;83:655-63. [PubMed]
- Zhan SS, Jiang JX, Wu J, et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 2006;43:435-43. [PubMed]
- Jiang JX, Mikami K, Venugopal S, et al. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol 2009;51:139-48. [PubMed]
- deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 2003;39:105-17. [PubMed]
- Mikula M, Proell V, Fischer AN, Mikulits W. Activated hepatic stellate cells induce tumor progression of neoplastic hepatocytes in a TGF-β dependent fashion. J Cell Physiol. 2006;209:560-7. [PubMed]
- Amann T, Bataille F, Spruss T, et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci 2009;100:646-53. [PubMed]
- Bandapalli OR, Geheeb M, Kobelt D, et al. Global analysis of host tissue gene expression in the invasive front of colorectal liver metastases. Int J Cancer 2006;118:74-89. [PubMed]
- Zhao W, Su W, Kuang P, et al. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol 2012;41:457-64. [PubMed]
- Zhao W, Zhang L, Xu Y, et al. Hepatic stellate cells promote tumor progression by enhancement of immunosuppressive cells in an orthotopic liver tumor mouse model. Lab Invest 2014;94:182-91. [PubMed]
- Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int 2014;34:834-43. [PubMed]
- Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol 2010;53:976-80. [PubMed]
- Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 2014;505:97-102. [PubMed]
- De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 2007;132:1937-46. [PubMed]
- Kristensen DB, Kawada N, Imamura K, et al. Proteome analysis of rat hepatic stellate cells. Hepatology 2000;32:268-77. [PubMed]
- Sancho-Bru P, Bataller R, Gasull X, et al. Genomic and functional characterization of stellate cells isolated from human cirrhotic livers. J Hepatol 2005;43:272-82. [PubMed]
- Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014;147:577-594.e1.
- Karlmark KR, Zimmermann HW, Roderburg C, et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010;52:1769-82. [PubMed]
- Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 2009;50:261-74. [PubMed]
- Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090-6. [PubMed]
- Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 2013;58:1461-73. [PubMed]
- Liaskou E, Zimmermann HW, Li KK, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology 2013;57:385-98. [PubMed]
- Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One 2010;5:e11049. [PubMed]
- Höchst B, Schildberg FA, Sauerborn P, et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol 2013;59:528-35. [PubMed]
- Aloman C, Tacke F. Dendritic cells in liver fibrosis: conductor of the inflammatory orchestra? Hepatology 2010;51:1070-2. [PubMed]
- Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta 2013;1832:1061-9.
- Wehr A, Baeck C, Heymann F, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol 2013;190:5226-36. [PubMed]
- Gur C, Doron S, Kfir-Erenfeld S, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut 2012;61:885-93. [PubMed]
- Glässner A, Eisenhardt M, Krämer B, et al. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest 2012;92:967-77. [PubMed]
- Eisenhardt M, Glässner A, Krämer B, et al. The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C. PLoS One 2012;7:e38846. [PubMed]
- Zimmermann HW, Mueller JR, Seidler S, et al. Frequency and phenotype of human circulating and intrahepatic natural killer cell subsets is differentially regulated according to stage of chronic liver disease. Digestion 2013;88:1-16. [PubMed]
- Syn WK, Agboola KM, Swiderska M, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut 2012;61:1323-9. [PubMed]
- Park O, Jeong WI, Wang L, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology 2009;49:1683-94. [PubMed]
- Chinnadurai R, Grakoui A. B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells. Hepatology 2010;52:2177-85. [PubMed]
- Jiang G, Yang HR, Wang L, et al. Hepatic stellate cells preferentially expand allogeneic CD4+ CD25+ FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation 2008;86:1492-502. [PubMed]
- Feng M, Wang Q, Wang H, et al. Adoptive transfer of hepatic stellate cells ameliorates liver ischemia reperfusion injury through enriching regulatory T cells. Int Immunopharmacol 2014;19:267-74. [PubMed]
- Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468-75. [PubMed]
- Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim Biophys Acta 2013;1832:876-83.
- Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 2014;14:181-94. [PubMed]
- Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A 2012;109:E3186-95. [PubMed]
- Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology 2014;59:1060-72. [PubMed]
- Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A 2012;109:9448-53. [PubMed]
- Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012;143:1073-83.e22.


