Immune mechanisms in acetaminophen-induced acute liver failure
Introduction
Acetaminophen (APAP) is a commonly used antipyretic and analgesic over-the-counter drug. Use of APAP is considered to be safe in therapeutic concentrations but can cause severe liver damage after an overdose, ultimately leading to acute liver failure (ALF) (1-3). Epidemiological studies revealed that about half of the cases of APAP overdoses were intentional (suicide attempts), mainly affecting young otherwise healthy individuals, while the other half of patients were unintentional overdosing, mostly in elderly patients taking more than one APAP-containing analgetics simultaneously for acute or chronic pain syndromes (1). The clinical outcome of APAP-induced ALF ranges from full recovery to need for liver transplantation or death (2). More recent studies on the pathogenesis of APAP-induced ALF revealed that not only AILI, but also subsequent inflammatory responses of the (predominantly innate) immune system critically determine the severity and outcome of disease (4). Several non-parenchymal cell types, cytokines and chemokines are incorporated in the inflammatory response following an acute injury of the liver. Besides resident hepatic macrophages, traditionally denoted to as Kupffer cell (KC), infiltrating monocyte-derived macrophage (MoMF), dendritic cell (DC) and different lymphoid derived immune cells are involved in AILI (5). Up to now only one specific pharmacological treatment option for patients suffering acetaminophen-induced liver injury (AILI) exists: the administration of high doses of the L-cysteine precursor N-Acetylcysteine (NAC). Unfortunately, the benefit of NAC administration decreases with the time passed between overdose and treatment (2). For later timepoints in severe cases of ALF, where inflammatory responses are more relevant, the only curative treatment option is liver transplantation with a survival rate of about 70% for the first 5 years (2). Considering the lack of potential donors and the high costs of a liver transplantation, there is an urgent need for alternative treatment options in the clinics. On account of these limitations, there has been increasing interest in understanding the immunological mechanisms underlying AILI. Therefore this review article will focus on the role of different immune cell subsets in the course of AILI and the potential targets to modulate the immune response for a better clinical outcome in this disease.
Initial damage of hepatocytes by APAP
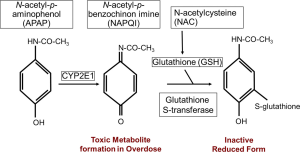
The underlying mechanism of AILI is the necrosis of hepatocytes. APAP is taken up from the intestine within the first few hours after consumption. A small fraction (10-15%) of APAP is metabolized in hepatocytes by cytochrome P450 isoforms into the alkylating metabolite N-acetyl-p-benzoquinone imine (NAPQI). The antioxidant glutathione (GSH) converts NAPQI into a harmless reduced form, which can then be excreted via the bile after glucuronidation (Figure 1). When GSH is depleted, due to an overdose of APAP, the increasing amount of NAPQI binds to mitochondrial proteins, forming cytotoxic protein adducts (2). The drug NAC is used in the synthesis of GSH, thereby replenishing the GSH level and giving a higher clearance for the toxic metabolite NAPQI (Figure 1).
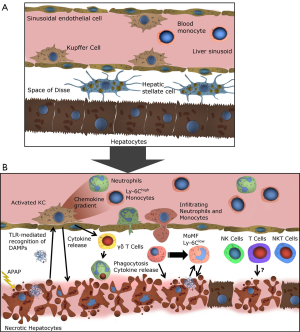
Two concepts have been stated to explain the ongoing necrosis of hepatocytes and the following activation of the immune system. In the ‘two-hit model’ the formation of protein adducts in hepatocytes leads to oxidative stress, with enhanced production of reactive oxygen species (ROS). ROS inhibit the pro-survival transcription factor NF-κB and subsequently trigger autophosphorylation and activation of c-Jun N-terminal kinase (JNK) (6). Activated JNK further enhances the ROS production, leading to mitochondrial membrane permeabilization and dysfunction (7). The mitochondrial dysfunction causes hepatocyte necrosis, followed by the release of various danger-associated-molecular patterns (DAMPs) (8). The most notable DAMPs in AILI are high-mobility group box protein 1 (HMGB-1), heat-shock protein-70 (HSP-70) and DNA fragments. DAMPs are recognized by KC, possibly also by other hepatic macrophages and by DC, via toll-like receptors (TLR), subsequently leading to the activation of these cell types (9). An important step in generating the inflammatory response is the formation of the inflammasome, a cytosolic multiprotein complex, in macrophages (10). After inflammasome formation IL-1β and IL-18 are secreted from KC, thereby further activating the innate immune system, leading to the recruitment of immune cells to the site of inflammation and advancing hepatocyte necrosis (Figure 2) (5). The alternative hapten model states that the presentation of NAPQI-protein adduct peptides by the human leukocyte antigen (HLA) on antigen presenting cells (APCs), leads to the activation of helper T (Th) cells and ultimately cytotoxic T (Tc) cells. Tc express surface FasL and release TNF-α, perforin and granzyme and are responsible for activation of death pathways in sensitized hepatocytes, thereby inducing necrosis (11). Necrotic hepatocytes passively release various mediators of inflammation, with DAMPs being the most notable, resulting in KC and subsequent innate immune cell activation as described above. The hapten model indicates a mechanism for AILI, which is different from other models of acute liver injury due to the specific involvement of HLA protein adduct presentation.
Immune responses following APAP-induced acute liver injury
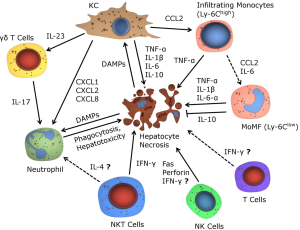
In order to explore the kinetics of immune cell recruitment and activation as well as the functional contribution of distinct leukocyte subpopulations, mouse models of AILI or (lethal) APAP-induced ALF have been investigated. Strikingly, total leukocytes in the liver increase about threefold 18 hours after APAP injection in mice (12), indicating a pronounced inflammatory reaction in the later course of AILI. In this review, we will therefore first present conclusive experimental evidence on immune mechanisms in AILI from mouse models (summarized in Figure 3), followed by translational data derived from patients suffering APAP-induced ALF.
Macrophages
Resident hepatic macrophages (KC) are seeded in the liver sinusoids, and act as a first line of defense of the innate immune system for the constant exposure to blood-borne food antigens and bacteria from the intestine (13). KC are eagerly involved in the mediation of liver injury by sensing for DAMPs and PAMPs (9), and the release of pro- and anti-inflammatory mediators such as TNF-α, IL-1β, IL-12, CCL2, CCL5, CXCL1, CXCL2, CXCL8, CXCL16 and IL-10 (14-17). In the early course of AILI, KC become depleted, while during the restorative phase they recover by self-renewal independent of M-CSF and infiltrating monocytes (18,19). In vitro studies could show that macrophages in AILI display increased metabolic and oxidative stress, finally leading to apoptosis of macrophages (20).
The exact role of KC for the progression of AILI is still controversial. The partial depletion and inhibition of KC by pretreatment with gadolinium chloride (GdCl3) attenuates liver injury, while mice with a fully-depleted KC population by using clodronate-loaded liposomes show aggravated liver injury (21). The reduced injury in GdCl3 treated mice has either been linked to the production of ROS (22), or to the expression of proinflammatory cytokines (21). However, the proposed direct cytotoxic effects of KC by producing ROS and peroxynitrite could not be confirmed by subsequent independent studies (23,24). The increased liver injury in mice completely lacking KC due to clodronate-loaded liposome treatment has been linked to reduced levels of IL-6, IL-10 and cyclooxygenase products (25). On the contrary, it has been shown that clodronate-loaded liposomes not only deplete KC, but also MoMF and circulating monocytes (26). Therefore, studies that used clodronate-loaded liposome treatment would potentially benefit from re-assessment, based on the recently revealed relationships between infiltrating monocytes, MoMF and KC (19). Further studies should therefore aim to use methodologies that allow a better lineage tracing and/or distinction between the different macrophage populations. Another explanation for increased liver damage after clodronate-loaded liposome treatment could be the priming towards inflammation by the massive depletion of cells itself, even before the application of APAP. Data from Holt et al. indicate that the population of MoMF is increased two days after the complete depletion of KC (27). On the other hand both GdCl3 and clodronate-loaded liposome treated mice show significantly enhanced survival rates in AILI compared to untreated controls independent of the differences in liver injury (21).
Recognition of DAMPs via TLRs by KC (e.g., HMGB1 by TLR4), leads to the production of pro-inflammatory cytokines (e.g., TNF-α, IL-1β and IL-6) (21) with TNF-α being highly relevant for sensitization of hepatocytes to apoptosis. In addition, hepatic macrophages also recruit other immune cells via the secretion of chemokines, e.g., CCL2 or CXCL16 of which the first recruits monocytes and the latter preferentially natural killer T (NKT) cells into areas of necrosis (27,28). Conversely, the secretion of IL-10 by KC attenuates ROS and peroxynitrite production, thereby counteracting the inflammatory condition in AILI (25,29).
Recently published results show that the upregulation of multi-drug resistant protein 4 (Mrp4) in hepatocytes is mediated by factors released by KC. The altered expression of transporters in hepatocytes is thought to be part of an self-protective response to AILI (30). In addition, studies could show that KC play an important role in resolution of liver injury by phagocytosis of apoptotic cells and cell debris and production of angiogenic factors and growth factors (25,31). The divergent role of KC in AILI might be due to distinct subpopulations which either enhance or counteract inflammation (17). Anti-inflammatory KC might be more susceptible to undergo apoptosis, which would further enhance inflammation and hepatocyte necrosis. Further investigations will be needed to clarify the role of the different KC populations in AILI.
While the amount of KC rapidly decreases during AILI (19), a high amount of infiltrating MoMF is recruited into areas of necrosis, mainly via CCL2/CCR2 (27). CCL2 also shows strong heterodimerization with CCL8, leading to synergistic effects in macrophage recruitment (32). MoMF are distinct from KC (27), as shown by differences in the gene expression pattern, whereas commonly used surface markers (like the macrophage marker F4/80) are equally expressed (19). Interestingly, MoMF are able to replace KC, if they are experimentally depleted (33). Infiltrating MoMF are considered immature, marked by a high expression of Ly6C (Gr1), and show a high expression of TNF-α, IFN-γ, IL-1β and IL-6 (17). Besides their pro-inflammatory cytokine profile, infiltrating monocytes and MoMF also secrete factors favoring angiogenesis, such as vascular endothelial growth factor (VEGF), and inhibit neutrophil recruitment and activation (19,34). In vitro studies with macrophages revealed that CCL2, in combination with IL-6, can promote the maturation of MoMF (35). Additional studies in a model of liver inflammation in rats could show that macrophages mature under the influence of CCL2 (36). To which extent these mechanisms are relevant in AILI in mice or in human remains still unclear. Mature MoMF are then characterized by a higher expression of the mannose receptor (CD206) and the anti-inflammatory cytokine IL-10 (17).
Infiltrating monocytes are strongly involved in the recovery phase by mediating angiogenesis and microvasculature remodeling by secretion of VEGF-A and matrix-metalloproteinases (MMP) (31). Besides mediating apoptosis of hepatocytes, TNF-α also induces proliferation of surviving hepatocytes as shown in other models of liver injury, which is a crucial step in the recovery from liver injury (37,38). Other chemokines connected to monocyte infiltration are CCL1, CCL25 and CX3CL1, which have been shown to play a role in other models of acute liver injury aside from AILI (39-41). Elucidating the role of these chemokines in the recruitment of macrophages in AILI might give further hints on different roles of functional macrophage subpopulations.
Furthermore, macrophages not only exert direct effects on liver parenchymal and non-parenchymal cells, but can also orchestrate responses from other immune cells such as NKT cells (28) or neutrophils (19).
Dendritic cells (DCs)
DCs are the major antigen-presenting cells in lymphoid organs and the periphery and are linked to innate and adaptive immune responses (42). Hepatic DC, in collaboration with KC, are considered important mediators of immune tolerance in the liver by suppressing CD4+ and CD8+ effector T cells and promoting regulatory T cells (43). The specific role of DC in the pathogenesis of AILI is at the moment obscure, which partly relates to the fact that several cells types in the liver can express ‘DC markers’ like MHC-II or CD11c. Following AILI DC are reported to show an increased expression of MHC-II, TLRs and produce a high amount of IL-6, CCL2 and TNF-α (44). Published results suggest that the depletion of DC leads to exacerbated liver injury, with a significantly higher expression of IL-6, CCL2 and TNF-α in serum, suggesting a protective role of DC in AILI. This protective effect of DC is, in contrary to KC, not linked to an increased IL-10 secretion, but might be due to reduced NK and neutrophil activation. However, the depletion of DC was achieved by a CD11c-diphteria toxin receptor model. In these models all CD11c+ cells are depleted, leaving the possibility that also other populations (macrophages, plasmacytoid DC) in the liver might have been influenced by this treatment (44). Moreover, depletion of DC via the CD11c-diphteria toxin might also have unspecific side effects such as neutrophilia, which may further impact the outcome of AILI (45). The individual contribution of different DC populations, like plasmacytoid dendritic cells (pDC), CD103+ or CD11b+ DC has not yet been elucidated in the context of AILI.
Neutrophils
Neutrophils are together with macrophages massively recruited into the liver upon AILI. Chemokine gradients of CXCL1, CXCL2 and CXCL8, primarily secreted by KC, guide neutrophils via CXCR1 and CXCR2 into the periphery of necrotic areas (46), whereas the chemotaxis into the inner site of necrosis is then mediated by a gradient of mitochondrial formyl peptides and the formyl peptide receptor 1 (FPR1) (47). However, it is currently unclear whether neutrophils directly aggravate the course of AILI. Different studies could show that a lack of neutrophils does not affect the outcome or severity of AILI (23,24,48). Additionally, invasive neutrophils are not significantly more activated compared to controls, as shown by equal CD11b expression and ROS priming (48). Initial results promoting a proinflammatory role of neutrophils (49), could later be linked to preconditioning protective effects on KC by the uptake of antibody tagged neutrophils (50). On the other side, recent studies indicated that neutrophils can directly mediate hepatocyte necrosis in AILI (51). In addition, infiltrating neutrophils upregulate TLR9, a receptor sensing extracellular DNA (51). Furthermore, TLR9-/- mice show attenuated inflammation during AILI, while the adoptive transfer of WT neutrophils in TLR9-/- mice reverted this hepatoprotective effect (52). These results underline the importance of recognizing free DNA following hepatocyte necrosis in AILI. Interestingly, elevated neutrophil levels sustained in the liver after acute injury, also suggesting a role in injury resolution (53). Taken together, neutrophils are a characteristic feature of immune responses in AILI, and diverging results concerning the role of neutrophils might be simply related to different experimental setting. Investigating the association between neutrophils and other resident or infiltrating immune cells, such as macrophages, might provide new insights in the participation of the innate immune system in AILI.
Natural killer (NK) cells
The liver is highly enriched in immune cells of the innate immune system, with NK cells being a main effector lymphocyte population (54). Upon activation, NK cells produce a variety of immunoregulatory cytokines such as IFN-γ, TNF-α and IL-10. In addition, NK cells can induce apoptosis of target cells via the release of perforin and granzyme (55). The role of NKs in AILI is still not fully elucidated. In principle, lymphoid cells contribute to the pathogenesis of AILI by producing IFN-γ and by depleting the GSH storages in hepatocytes via the Fas/FasL system (56). IFN-γ and FasL expression are two well-known effector mechanisms of hepatic NK and NKT cells (57). However, other studies suggesting a direct pathogenic role for NK and NKT cells upon AILI (58) could later be linked to the use of dimethyl-sulfoxide (DMSO) as a solvent. It was shown that DMSO alone leads to the activation of NK and NKT-cells, characterized by an increased expression of IFN-γ and granzyme-B (59). Nonetheless, these studies revealed a role of IFN-γ in the pathogenesis of AILI. Mice treated with IFN-γ neutralizing antibodies, or IFN-γ deficient knockout mice show attenuated hepatotoxicity following APAP overdose (60). The question of the origin of the IFN-γ could not be answered by these results. It is also conceivable that NKT or T cells secrete high amounts of IFN-γ.
Classical T cells
Secondary to innate immune cells, lymphocytes belonging to adaptive immunity, such as CD4 T helper (Th) and CD8 cytotoxic T (Tc) cells are recruited into the liver during the course of AILI. Th cells can be subdivided into four distinct subpopulations: Th1, Th2, Th17 and regulatory T (Treg) cells. Th1 cells are characterized by pro-inflammatory cytokine expression (e.g., IFN-γ), whereas Th2 cells are linked to the expression of IL-4, IL-5 and IL-13 (61). The role of Th cells in AILI was investigated by using two different mouse strains with different susceptibility to either Th1 or Th2 responses. C57BL/6 mice, associated with a dominant Th1 response, and Balb/c mice, which develop predominantly a Th2 response, were treated with APAP. C57BL/6 mice showed enhanced liver injury and increased expression of TNF-α in comparison to Balb/c mice (62). This might either indicate a specific pathogenic role for Th1 cells in AILI, or could possibly also be related to an “M1-like” macrophage polarization as suggested by other studies using a model of non-alcoholic steatohepatitis (NASH) (63). In this context, results mentioned above showing an attenuated liver injury by the use of an IFN-γ antibody, could also be due to a reduced Th1 response and not related to NK cells (60). IL-17 secreting cells are found as subsets of CD4 (Th17) and CD8 (Tc17) cells. Th17 cells develop under the control of the transcription factor RORγt, in response to antigens and in combination with IL-6 and TGF-β. Th17 cells are generally associated with inflammation and autoimmunity. Under inflammatory conditions infiltrating IL-17+ T cells are recruited into the liver sinusoids via CXCR3 (64). Their specific role in AILI has not been investigated yet.
Non-classical T cells
NKT cells
NKT cells are a subpopulation of T cells, which additionally express surface markers and cytokines characteristic for NK cells. Under homeostatic conditions NKT cells are the most abundant lymphocyte population in the liver that crawl along the sinusoids (65). NKT cells can be further divided into type I and II NKT cells of which type I NKT cells (invariant NKT or iNKT) represent the major subset. NKT cells are activated by glycosphingolipid recognition via the non-polymorphic major histocompatibility complex class-I like molecule (CD1d) (66). In models of liver fibrosis NKT cells are infiltrating into the areas of necrosis via the CXCL16/CXCR6 axis, thereby further enhancing inflammation (28). In ConA-mediated experimental hepatitis NKT cells seem to aggravate the liver injury (65). In α-galceramide (α-Galcer) mediated hepatitis activated invariant NKT cells rapidly release IL-4, which enhances survival of neutrophils, thereby aggravating liver injury. The rapid release of IL-4 is then followed by production of IFN-γ, acting as a negative regulator by promoting apoptosis of neutrophils (67). Studies using mouse models with genetic deletion of NKT cells (CD1d-/- and Jα18-/-) show that NKT deficient mice are more susceptible to AILI. This increased susceptibility to APAP seems to be associated with an increased expression of CYP2E1 during starvation, resulting in enhanced NAPQI-protein adduct formation compared to WT control mice. If APAP is applied to mice without starvation this effect is blunted (68). It still remains unclear whether NKT cells play a major role in the later course of AILI. NKT cells might either be involved in AILI by changing the cytochrome P expression, or by secreting IL-4 and IFN-γ, thereby changing the inflammatory milieu in necrotic areas.
Gamma-delta (γδ) T cells
γδ T cells are a unique subpopulation of T cells, being especially enriched in liver tissue. γδ T cells are thought to link the innate and the adaptive immune system, as they show characteristics of both, and additionally own the capacity to secrete a variety of immunomodulatory mediators. γδ T cells can be further segregated into either IFN-γ or IL-17 expressing subtypes, which might be a reason for their either protective or pro-inflammatory role in different liver diseases (69,70). Recent results show that IL-23 secretion of activated macrophages, which stimulates the IL-17 γδ T cell subpopulation, leads to the accumulation of neutrophils into areas of necrosis and enhanced inflammation. Vice versa, depletion of γδ T cells significantly reduced IL-17A production after AILI (71). These results align with recent findings suggesting an involvement of neutrophils in the early course of AILI (51). Therefore, γδ T cells serve as orchestrators in the early onset of the inflammatory response in AILI by their ability to recruit neutrophils. It still remains unclear whether γδ T cells could also be directly involved to AILI themselves.
Translation into human pathogenesis and potential therapeutic targets
Increasing knowledge of the underlying immunological pathways following AILI from mouse models gives rise to the expectation of exploring new treatment options for ALF in humans. Up to date the only available specific pharmacological treatment for AILI in patients is NAC, and no drugs targeting the APAP-induced inflammatory responses have been approved yet. Nevertheless, there exist several substances that have been tested in mice, of which some might bear potential as a treatment option in the future. One problem is that AILF is often associated with a systemic inflammatory response, which dramatically reduces the survival of patients (72). Many substances that have been described to attenuate AILI focus on cytochrome p450 and have therefore no therapeutic value for later time-points. Table 1 summarizes the main immune cells and their mediators in AILI as well as potential options for therapeutic targets.
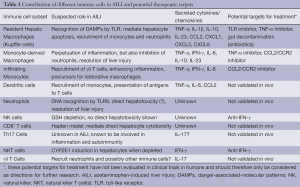
Full table
The main focus on changing the course of AILI has been laid on hepatic macrophages and infiltrating monocytes. Monocytes in humans can be divided into three distinct subpopulations by the surface markers CD14 and CD16. These are: CD14++/CD16– ‘classical’ monocytes, CD14+/CD16+ ‘non-classical’ monocytes and CD16++ atypical monocytes and DCs. Human CD14++/CD16- monocytes are similar to Ly-6Chigh in mice and CD14+/CD16+ to Ly-6Clow monocytes, respectively (17). Regarding the role of macrophages in human AILI, Antoniades et al. have shown that in consistency with findings from experimental models, the hepatic macrophage population rapidly expands after an APAP overdose (4). The increase of hepatic macrophages is based on proliferation of local KC and the infiltration of monocytes into areas of necrosis. In men as in mice, CCL2 was shown to be the mediator of monocyte egress from the bone-marrow into circulation (4,27). Infiltrating human monocytes are marked by a high expression of MAC387+ (4). A positive outcome for AILI patients is associated with a high number of circulating monocytes and low serum levels of CCL2, indicating that a fast and high-numbered infiltration of monocytes into APAP-injured liver might be disadvantageous for the survival of the patients. At later time points, infiltrating macrophages become tightly involved in resolution of inflammation and repair (4).
The severity of liver damage seems to be correlated to the balance of MoMF and resident KC. Thus, inhibiting the infiltration of immature macrophages could bear therapeutic potential. In line with this hypothesis, the CCL2 inhibitor mNOX-E36 (Noxxon Inc., Berlin, Germany) has been used in experimental models of liver fibrosis and inhibited the infiltration of Ly-6Chigh macrophages, resulting in accelerated regression (73,74). On the other side, blockade of CCL2 was recently shown to induce a M1-like gene expression profile in human macrophages in vitro (35). Pharmacological inhibition of infiltrating monocytes would need to be timed accurately, in order to reduce the onset of inflammation and the risk for ALF. In addition, any therapeutic approach would need to focus on not completely inhibiting infiltrating monocytes, as it has been shown that the complete absence of MoMF impairs the recovery from liver injury (31,75).
Aside from inhibiting monocytes and macrophages, blocking of pro-inflammatory cytokines is another potential therapeutic approach. Patients suffering from AILI show higher serum levels of IL-4, IL-6, IL-10 and TNF-α (76). Regarding the central role of TNF-α in AILI a possible therapeutic approach could be the use of infliximab, a specific TNF-α inhibitor. Infliximab has been shown to have hepatoprotective activity in a model of AILI in rats (77). However, due to its strong immune-suppressive activities, anti-TNF based therapies could be potentially harmful in patients with established systemic inflammatory response syndrome, as infectious complications are a life-threatening risk for patients with ALF (78,79). Another approach might be the use of IL-22 in combination with NAC, which could improve the overall outcome of patients with severe cases of AILI by increasing hepatocyte proliferation (80).
Altering the recognition of DAMPs could be another promising strategy in reducing the severity of AILI. Besides from activating the immune system, HMGB1 released by necrotic hepatocytes also leads to increased gut permeability and bacterial translocation from the intestines, ultimately promoting multi-organ dysfunction and sepsis. Inhibition of HMGB1 in mice attenuates liver injury and reduces bacterial translocation (81). Specific TLR4 antagonists could also be used to reduce the activation of immune cells, such as KC, thereby ameliorating AILI (82). Administration of the non-absorbable antibiotic rifaximin might reduce bacterial translocation, production of ammonia by gut bacteria and, to some extent, other toxic derivatives from the gut, as evidenced from studies from hepatic encephalopathy in patients with chronic liver diseases (83). In addition, also the use of corticosteroids has been proposed as potentially beneficial by suppressing the overboarding immune reaction following AILI. Preclinical studies using corticosteroids in AILI could show that glucocorticoids seem to sensitize mice towards AILI, while the inhibition of glucocorticoid receptors was found to be protective (84). A recent multi-center clinical trial investigating the use of corticosteroids in patients with drug-induced ALF suggested no benefit from using corticosteroids on the overall outcome (85).
Another approach focuses on the role of DNA pattern recognition. It has been shown that mitochondrial DNA and CXCL2 are elevated in serum of ALF patients (51). TLR9 can activate neutrophils by binding CpG-rich DNA, thereby enhancing inflammation in the liver (52). The recognition of DNA by TLR9 in other organs leads to a systemic inflammatory response, which is often found in AILI patients. Interesting in this context is the finding, that a novel TLR9-antagonist ameliorates sterile inflammation-induced organ damage (86), which might be also useful in treating ALF. Whether DNA recognition by neutrophils represents one of the main triggers for ongoing hepatic injury, however, remains unclear. Up to present no direct correlation, for the level of neutrophils and the outcome of AILF in human, could be shown (4). Only a few studies focused on the role of T cells in human AILI. It is known that during AILI T cells are recruited into the liver by upregulation of adhesion molecules and chemokines of the hepatic parenchyma. Recruited T cells show an upregulated phenotype characterized, for instance, by a higher expression of the chemokine receptor CXCR3 (87). However, this has not been translated into therapeutic approaches targeting T cells in AILI.
Conclusions
Recent findings on the pathogenesis of APAP induced acute liver injury and liver failure revealed that the outcome of the insult not only depends on the direct hepatotoxic effects of the noxious metabolite NAPQI, but is critically dependent on the complex interplay of different immune cells constituting inflammatory responses to AILI. An incontestable fact is the necrosis of hepatocytes followed by a massive infiltration of macrophages, neutrophils and different lymphoid cell types, which aggravate or decrease the inflammation in areas of necrosis. Most of the data dissecting the essential contributions especially of innate immune cells like KC, monocytes, neutrophils and NK cells are generated in experimental rodent models, because targeted interventions in experimental AILI allow dissecting the complexity of the immune mechanisms in AILI. It is now of utmost importance to translate findings from mouse models in human pathogenesis and identify the most promising targets for future interventional studies, aiming at limiting overwhelming inflammatory responses in the liver and improving outcome of APAP-induced ALF in patients.
Acknowledgements
The authors thank all members of the Tacke lab and the Medical Clinic III for helpful discussions. This work was supported by the German Research Foundation (DFG Ta434/2-1, DFG SFB/TRR57) and by the Interdisciplinary Center for Clinical Research (IZKF) Aachen.
Disclosure: The authors have received reagents from Noxxon Inc. (Berlin, Germany) and Tobira Therapeutics Inc. (San Francisco, CA, USA) for experimental research targeting monocytes/macrophages.
References
- Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364-72. [PubMed]
- Larsen FS, Wendon J. Understanding paracetamol-induced liver failure. Intensive Care Med 2014;40:888-90. [PubMed]
- Chun LJ, Tong MJ, Busuttil RW, et al. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol 2009;43:342-9. [PubMed]
- Antoniades CG, Quaglia A, Taams LS, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012;56:735-46. [PubMed]
- Jaeschke H, Williams CD, Ramachandran A, et al. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int 2012;32:8-20. [PubMed]
- Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 2008;283:13565-77. [PubMed]
- Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem 2011;286:16052-62. [PubMed]
- Dahlin DC, Miwa GT, Lu AY, et al. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A 1984;81:1327-31. [PubMed]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 2010;192:387-94. [PubMed]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology 2012;143:1158-72. [PubMed]
- Han D, Shinohara M, Ybanez MD, et al. Signal transduction pathways involved in drug-induced liver injury. Handb Exp Pharmacol 2010:267-310.
- Sanz-Garcia C, Ferrer-Mayorga G, González-Rodríguez Á, et al. Sterile inflammation in acetaminophen-induced liver injury is mediated by Cot/tpl2. J Biol Chem 2013;288:15342-51. [PubMed]
- Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol 2012;3:56. [PubMed]
- Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014;147:577-594.e1.
- Luedde T, Schwabe RF. NF. -κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011;8:108-18. [PubMed]
- Gardner CR, Hankey P, Mishin V, et al. Regulation of alternative macrophage activation in the liver following acetaminophen intoxication by stem cell-derived tyrosine kinase. Toxicol Appl Pharmacol 2012;262:139-48. [PubMed]
- Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090-6. [PubMed]
- Dambach DM, Watson LM, Gray KR, et al. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 2002;35:1093-103. [PubMed]
- Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol 2014;193:344-53. [PubMed]
- Al-Belooshi T, John A, Tariq S, et al. Increased mitochondrial stress and modulation of mitochondrial respiratory enzyme activities in acetaminophen-induced toxicity in mouse macrophage cells. Food Chem Toxicol 2010;48:2624-32. [PubMed]
- Fisher JE, McKenzie TJ, Lillegard JB, et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res 2013;180:147-55. [PubMed]
- Michael SL, Pumford NR, Mayeux PR, et al. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999;30:186-95. [PubMed]
- Cover C, Liu J, Farhood A, et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 2006;216:98-107. [PubMed]
- James LP, McCullough SS, Knight TR, et al. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res 2003;37:1289-97. [PubMed]
- Ju C, Reilly TP, Bourdi M, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol 2002;15:1504-13. [PubMed]
- Tacke F, Ginhoux F, Jakubzick C, et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med 2006;203:583-97. [PubMed]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol 2008;84:1410-21. [PubMed]
- Wehr A, Baeck C, Heymann F, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol 2013;190:5226-36. [PubMed]
- Bourdi M, Masubuchi Y, Reilly TP, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 2002;35:289-98. [PubMed]
- Campion SN, Johnson R, Aleksunes LM, et al. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am J Physiol Gastrointest Liver Physiol 2008;295:G294-304. [PubMed]
- You Q, Holt M, Yin H, et al. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol 2013;86:836-43. [PubMed]
- Crown SE, Yu Y, Sweeney MD, et al. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J Biol Chem 2006;281:25438-46. [PubMed]
- Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 2007;110:4077-85. [PubMed]
- Ehling J, Bartneck M, Wei X, et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 2014;63:1960-71. [PubMed]
- Sierra-Filardi E, Nieto C, Domínguez-Soto A, et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 2014;192:3858-67. [PubMed]
- Mori Y, Izawa T, Takenaka S, et al. Participation of functionally different macrophage populations and monocyte chemoattractant protein-1 in early stages of thioacetamide-induced rat hepatic injury. Toxicol Pathol 2009;37:463-73. [PubMed]
- Meijer C, Wiezer MJ, Diehl AM, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 2000;20:66-77. [PubMed]
- Shuh M, Bohorquez H, Loss GE Jr, et al. Tumor Necrosis Factor-α: Life and Death of Hepatocytes During Liver Ischemia/Reperfusion Injury. Ochsner J 2013;13:119-30. [PubMed]
- Heymann F, Hammerich L, Storch D, et al. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology 2012;55:898-909. [PubMed]
- Nakamoto N, Ebinuma H, Kanai T, et al. CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology 2012;142:366-76. [PubMed]
- Karlmark KR, Zimmermann HW, Roderburg C, et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010;52:1769-82. [PubMed]
- Plitas G, Burt BM, Stableford JA, et al. Dendritic cells are required for effective cross-presentation in the murine liver. Hepatology 2008;47:1343-51. [PubMed]
- Crispe IN. Immune tolerance in liver disease. Hepatology 2014;60:2109-17. [PubMed]
- Connolly MK, Ayo D, Malhotra A, et al. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology 2011;54:959-68. [PubMed]
- Tittel AP, Heuser C, Ohliger C, et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods 2012;9:385-90. [PubMed]
- Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets 2011;10:509-36. [PubMed]
- McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010;330:362-6. [PubMed]
- Williams CD, Bajt ML, Farhood A, et al. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int 2010;30:1280-92. [PubMed]
- Liu ZX, Han D, Gunawan B, et al. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006;43:1220-30. [PubMed]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology 2007;45:1588-9. [PubMed]
- Marques PE, Amaral SS, Pires DA, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012;56:1971-82. [PubMed]
- Marques PE, Oliveira AG, Pereira RV, et al. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology 2014. [Epub ahead of print]. [PubMed]
- Williams CD, Bajt ML, Sharpe MR, et al. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol 2014;275:122-33. [PubMed]
- Godfrey DI, Hammond KJ, Poulton LD, et al. NKT cells: facts, functions and fallacies. Immunol Today 2000;21:573-83. [PubMed]
- Schuch A, Hoh A, Thimme R. The role of natural killer cells and CD8(+) T cells in hepatitis B virus infection. Front Immunol 2014;5:258. [PubMed]
- Tinel M, Berson A, Vadrot N, et al. Subliminal Fas stimulation increases the hepatotoxicity of acetaminophen and bromobenzene in mice. Hepatology 2004;39:655-66. [PubMed]
- Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol 2009;36:4-12. [PubMed]
- Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004;127:1760-74. [PubMed]
- Masson MJ, Carpenter LD, Graf ML, et al. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology 2008;48:889-97. [PubMed]
- Ishida Y, Kondo T, Ohshima T, et al. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J 2002;16:1227-36. [PubMed]
- Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol 2011;2011:345803.
- Masubuchi Y, Sugiyama S, Horie T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem Biol Interact 2009;179:273-9. [PubMed]
- Maina V, Sutti S, Locatelli I, et al. Bias in macrophage activation pattern influences non-alcoholic steatohepatitis (NASH) in mice. Clin Sci (Lond) 2012;122:545-53. [PubMed]
- Zhu X, Uetrecht J. A novel T(H)17-type cell is rapidly increased in the liver in response to acetaminophen-induced liver injury: T(H)17 cells and the innate immune response. J Immunotoxicol 2013;10:287-91. [PubMed]
- Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 2005;3:e113. [PubMed]
- Mossanen JC, Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology 2013;2:e26468. [PubMed]
- Wang H, Feng D, Park O, et al. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-γ. Hepatology 2013;58:1474-85. [PubMed]
- Martin-Murphy BV, Kominsky DJ, Orlicky DJ, et al. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology 2013;57:1575-84. [PubMed]
- Hammerich L, Tacke F. Role of gamma-delta T cells in liver inflammation and fibrosis. World J Gastrointest Pathophysiol 2014;5:107-13. [PubMed]
- Serre K, Silva-Santos B. Molecular Mechanisms of Differentiation of Murine Pro-Inflammatory γδ T Cell Subsets. Front Immunol 2013;4:431. [PubMed]
- Wang X, Sun R, Wei H, et al. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of γδ T cells with macrophages. Hepatology 2013;57:373-84. [PubMed]
- Rolando N, Wade J, Davalos M, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000;32:734-9. [PubMed]
- Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology 2014;59:1060-72. [PubMed]
- Baeck C, Wehr A, Karlmark KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012;61:416-26. [PubMed]
- Possamai LA, Thursz MR, Wendon JA, et al. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol 2014;61:439-45. [PubMed]
- Antoniades CG, Berry PA, Davies ET, et al. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology 2006;44:34-43. [PubMed]
- Ferah I, Halici Z, Bayir Y, et al. The role of infliximab on paracetamol-induced hepatotoxicity in rats. Immunopharmacol Immunotoxicol 2013;35:373-81. [PubMed]
- Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol 2012;57:1336-48. [PubMed]
- Bernal W, Auzinger G, Sizer E, et al. Intensive care management of acute liver failure. Semin Liver Dis 2008;28:188-200. [PubMed]
- Scheiermann P, Bachmann M, Goren I, et al. Application of interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am J Pathol 2013;182:1107-13. [PubMed]
- Yang R, Zou X, Tenhunen J, et al. HMGB1 neutralization is associated with bacterial translocation during acetaminophen hepatotoxicity. BMC Gastroenterol 2014;14:66. [PubMed]
- Shah N, Montes de Oca M, Jover-Cobos M, et al. Role of toll-like receptor 4 in mediating multiorgan dysfunction in mice with acetaminophen induced acute liver failure. Liver Transpl 2013;19:751-61. [PubMed]
- Garcovich M, Zocco MA, Roccarina D, et al. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol 2012;18:6693-700. [PubMed]
- Masson MJ, Collins LA, Carpenter LD, et al. Pathologic role of stressed-induced glucocorticoids in drug-induced liver injury in mice. Biochem Biophys Res Commun 2010;397:453-8. [PubMed]
- Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology 2014;59:612-21. [PubMed]
- Hoque R, Farooq A, Malik A, et al. A novel small-molecule enantiomeric analogue of traditional (-)-morphinans has specific TLR9 antagonist properties and reduces sterile inflammation-induced organ damage. J Immunol 2013;190:4297-304. [PubMed]
- Tuncer C, Oo YH, Murphy N, et al. The regulation of T-cell recruitment to the human liver during acute liver failure. Liver Int 2013;33:852-63. [PubMed]


