Therapeutic targeting of liver inflammation and fibrosis by nanomedicine
Liver biology, innovative drugs, and arising hope from nanomedicine
The liver is the largest internal human organ weighing about 1.5 kg in an adult, with many essential roles in metabolism and clearance. Bile acids are formed by the liver, which are of critical importance for the maintenance of cholesterol metabolism and intestinal lipid absorption. The capability of the liver to regenerate is remarkable, because it can even compensate a loss of 70% of the parenchyma by proliferation within several weeks (1). However, sustained injury promotes a characteristic wound healing response termed fibrosis, in which the tissue is encircled by deposited extracellular matrix (ECM) proteins and undergoes severe structural and functional alterations (2). Chronic liver diseases represent a major health concern worldwide, with chronic viral hepatitis, metabolic disorders, malnutrition, alcohol abuse, or autoimmune diseases being major causes of chronic liver injury and subsequent complications such as liver cirrhosis or hepatocellular carcinoma (3).
Numerous innovative attempts of treating liver diseases such as interferon γ (IFNγ) (4), angiotensin II antagonists (5), and interleukin 10 (IL10) (6) have yielded promising results in preclinical trials, but the majority of these approaches were not successful in clinical trials. One of the reasons is likely that the delivery of the respective molecules by traditional formulations is not specific enough. For instance, IFNγ can have potent antifibrotic activities, but has proinflammatory effects on macrophages (7), if no cell-specific targeting is achieved. Nanomaterial-based drugs may overcome many of the hurdles of traditional non-nano drugs, because they bear the advantage of enabling a cell-type specific drug delivery based on binding to a specific surface structure. Cell-specificity increases the concentration at the critical target cell or tissue, while reducing a putative toxicity for other cell types. This is an important feature of nanodrugs, since many common drugs have limited efficacy because their concentration at the target site is too low. Additionally, nanosystems may overcome biological barriers based on their sizes, protect the drug from being metabolized, facilitate a delivery of otherwise undeliverable drugs, enable a prolonged drug release, and alter the pharmacological features of the transmitted drug (8-11).
Classifications of nanoparticles
Therapeutic nanoparticles range from 1 to 100 nm (12), however, many carrier systems require larger sizes for drug release and thus frequently, the definition is being extended to the submicron scale (up to 500 nm). Basically, nano-sized particulates can be assigned into two major groups that refer to its organic or inorganic nature. The most prominent inorganic nanoparticles are gold nanoparticles (AuNP), which can be rather easily modified in size, shape, and functionalization such as nanorods (13), nanocages (14), and nanostars (15). Metal core nanoparticles exhibit characteristic physicochemical properties that provide optical and magnetic properties to allow their usage in anatomical, cellular, and molecular imaging (16,17). However, the inorganic nanoparticles have the disadvantage that they accumulate in the body since they are not biodegradable. We have shown earlier that gold nanorods (AuNRs) remain in the liver at similar levels even after seven days compared to day one after injection (16).
The broad spectrum of organic nanoparticles includes liposomes and those on a polymer base such as N-(2-Hydroxypropyl) methacrylamide (HPMA) (18). The organic nanoparticles exhibit the big advantage that many of them are biodegradable. For instance, liposomes can simply fuse with cell membranes due to their similar composition. On the contrary, some organic nanoparticles are not biodegradable such as fullerenes or carbon nanotubes (19).
Distribution and toxicity of nanoparticles in the liver
The particle material, size, and putative functionalizations determine the distribution in different organs and cells. The accumulation of nanoparticles in different organs depends on their size. When AuNP of the sizes 10, 50, 100, and 250 nm were injected in rodents, most gold was present at liver and spleen. Only the smaller 10 nm sizing nanoparticles were found widespread in many other organs such as kidney, testis, and brain (20), similar to results of other studies, in which 15 nm nanospheres were also more widely distributed than larger particles. We found earlier that accumulation in liver and spleen also holds true for AuNRs sizing 50 nm × 15 nm (16). The exceptional capacity of the liver for nanoparticle clearance is partly explained by its enormous size compared to the spleen as the dry weight of the liver is about 50-fold higher than that of spleen (16). Data for the biodistribution of magnetic iron oxide-based nanoparticles are similar to those of AuNP, mea nm ning that iron oxide-based nanoparticles with a core size of 11 nm were also rather widely distributed in the body (21). However, also nanoparticle engulfing chemicals such as polyethylene glycol (PEG) that are designed to increase the circulation time of nanomaterials accumulate in the liver. The regular accumulation in the liver makes nanoparticles ideal candidates for treating hepatic diseases upon parenteral administration, but also puts them into focus for the toxicity of therapeutic strategies that do not intend to target the liver.
Another reason for the predominant accumulation of nanoparticles in the liver may be related to the high number of macrophages in the liver, which contains approximately 80-90% of all macrophages of the body (22). The nanoparticle charge is important for interactions with immune cells, and positive charges increase the particle uptake by immune and endothelial cells (23), meaning by cells of the reticuloendothelial system. The extracellular immobilization of nanoparticles by human immune cells via extracellular traps also depends on the charge of the particles (24). In a detailed investigation using quantum dots as model particles, zwitterionic or neutral organic coatings prevented the adsorption of serum proteins on nanoparticles, which play an important role for nanoparticle uptake as they determine and potentially increase the hydrodynamic diameter of nanoparticles, which is decisive for their renal filtration. The size of 15 nm was shown to be the threshold for renal clearance of nanoparticles, and a particle diameter below 5.5 nm leads to an increased renal excretion (17).
Nanoparticles like AuNP are generally non-toxic (16), similar to other inorganic such as magnetic nanoparticles (21). However, small AuNP exhibiting a size of 1.4 nm were shown to be toxic (25). Nevertheless, at high doses, also many nanotherapeutics might turn toxic as demonstrated for titanium dioxide nanoparticles (26). Silica-based nanoparticles were shown to exhibit toxicity by activating macrophages (27,28). To prevent the attachment with serum proteins, many nanoparticles including gold-based systems are PEGylated to reduce their unspecific uptake by phagocytes by evoking a neutral charge (29). Additionally, nanoparticles can be functionalized with peptides (23), which may alter the response of immune cells such as those of macrophages and dendritic cells (16,23,30).
Four major cell types in the liver under healthy and disease conditions
For clarity reasons, we focus this review on four major cell types in the liver, hepatocytes as the major parenchymal cells, and on the three major non-parenchymal cell types hepatic stellate cells (HSC), macrophages, and LSEC, because all these cell types are critically involved in liver disease progression and might be promising cellular targets for nanomedicine. Nevertheless, the full picture of hepatic cells is much more complex, with additional parenchymal cells (such as cholangiocytes), stem cell compartments (for example oval cells) and manifold patrolling or tissue-resident immune cells (specifically natural killer T cells, monocytes, T lymphocytes and many others).
The majority of the liver consists of parenchymal cells, mostly hepatocytes, which can form 80% of the liver volume, while the non-parenchymal cells represent 40% of the total number of liver cells but only 6.5% of the organ volume. The lifespan of hepatocytes was shown to be 200 days in mice (31) and 400 days in rats (32), which reflects the continuous process of self-renewal within the liver. Under healthy conditions, hepatocytes fulfil many of the key functions of the liver such as protein synthesis and storage, carbohydrate turnover, synthesis of bile salts, phospholipids, and cholesterol, detoxification, modifications, and eliminations of exogenous and endogenous substances. During liver disease, which can be triggered by different injuries such as viral infections, metabolic syndrome or excessive alcohol intake, hepatocytes undergo apoptosis (33), and in the following are replaced with ECM, a process involved in the key event of liver fibrogenesis (Figure 1).
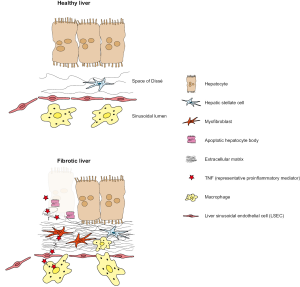
HSC are located in the perisinusoidal space (the area between the sinusoids and hepatocytes), which is also known as the space of Dissé. They usually represent 5-8% of the total number of liver cells, and their protrusions engulf the sinusoids (34). In healthy individuals, quiescent HSC store vitamin A in the liver and secrete a limited amount of ECM proteins (34). During the course of liver disease, HSC undergo a transdifferentiation into myofibroblasts, which are highly proliferative and produce large amounts of ECM proteins such as collagen type I and III, which leads to an excess production of hepatic connective tissue, termed fibrosis, and a reduction in liver functionality (35). The tonic contraction of activated HSC leads to increased liver stiffness, which is one hallmark of liver fibrosis and cirrhosis. HSC activation can be triggered by innate hepatic immune cells, especially by macrophages (36). Macrophages sense pathogenic threats based on the recognition of pathogen-associated molecular patterns (PAMP) or damage-associated molecular patterns (DAMP), which both lead to an activation of the inflammasome complex in macrophages (37). Upon activation of the inflammasome, macrophages secrete large amounts of cytokines that can activate HSC and other cell types (35) (Figure 1).
Macrophages are an essential cell population in the liver for maintaining tissue homeostasis, but also for the response to injury and progression of liver disease. Macrophages in the liver are a surprisingly heterogeneous population, which differs with respect to cellular origin, activation state and functional properties (38). One of the predominant macrophage populations of the liver are the Kupffer cells (KC), which originate from local precursors, have high phagocytic activity, easily respond to “danger signals” and maintain immune homeostasis in non-inflamed conditions (38-40). KC are rather stationary and immotile cells (39), in contrast to inflammatory macrophages (iMΦ) in the liver, which originate from circulating monocytes and massively infiltrate the liver upon injury (41). In a normal healthy liver, the KC function as immune sentinels that activate other cells and components of the immune system upon pathogenic threats (22,42).
Nanomaterial uptake was shown to alter the state of macrophage activation (also termed polarization) and thereby to affect the profile of their cytokine release in vitro and in vivo (16,23). Cytokine levels are important mediators of tissue injury and inflammation and can therefore also be considered as biomarkers for disease activity. Their presence in the serum can affect inflammatory reactions in the whole body, including the central nervous system and are also symptomatic for Alzheimer's disease and vascular dementia (43). Cytokines can further affect various cell types (44) and tissues (45).
The inflammatory cytokines released by macrophages can be assigned into different categories reaching from pro to antiinflammatory (29). Although there is a broad spectrum of macrophage activation depending on factors from the inflamed environment (46), macrophage polarization is often simplified into proinflammatory M1 and antiinflammatory M2 cells. Alterations in the balance between both subtypes appear in different diseases, and macrophages are typically skewed towards the M1 subtype in proinflammatory diseases, whereas M2 cells appear in cancer, allergy, and at late stages of inflammation. IL1β and TNF are two essential proinflammatory cytokines that affect nearly any inflammatory disease. Antiinflammatory cytokines directly suppress proinflammatory mediators, the most important antiinflammatory cytokine IL10 suppresses IL6 and TNF production (Figure 1) (47).
Liver sinusoidal endothelial cells (LSEC) constitute about half of the nonparenchymal cells of the liver. LSEC separate the hepatocytes from the blood of the sinusoidal lumen. This endothelial cell layer lacks a basement membrane and has fenestrae (also called sieve plates) sizing 100 nm, which permit the passage of molecules smaller than 100 nm from the sinusoidal lumen into the space of Dissé (48). LSEC share the expression of a large number of receptors with macrophages, such as pattern recognition receptors (PRR), specifically the Toll-like receptors (TLR) 3, 4, 7, and 9 (49,50). Further, LSEC were shown to internalize antigen, cellular debris, and immune complexes (49,51-53), sizing up to 1 µm in diameter (53). Based on their internalizing capabilities, LSEC can deliver molecules up to 1 µm to hepatocytes and HSC, either through the fenestrae or via transcytosis (39). LSEC were also shown to act as antigen-presenting cells and to be able to present antigen directly to T cells (54) (Table 1).
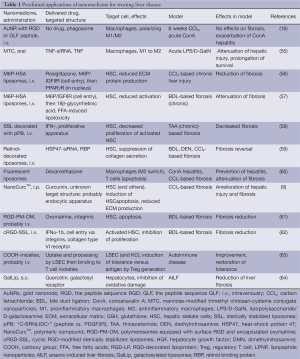
Full table
Under normal conditions, healthy LSEC protect the HSC from activation and can even deactivate activated HSC (65). Upon liver injury, LSEC show a lack of differentiation, a process that precedes fibrosis and is called capillarization. This process is reversible, but, however, does not reverse fibrogenesis upon inhibition (66). Following their capillarization, LSEC lose their fenestrations and enable macrophages and other immune cells to infiltrate the space of Dissé (Figure 1).
Liver cell targeting using nanomedicine
Different strategies have been proposed and tested in the preclinical setting to target the above-mentioned four major cell types in healthy and diseased livers. Besides modifying the physical properties of the nanomaterial (chemical structure, size, charge, conformation), surface markers or molecules of the liver cell types have been used to identify and to target them specifically using antibodies or receptor ligands that bind to these structures (Figure 2), selected highlights of cell targeting are shown in Table 1.
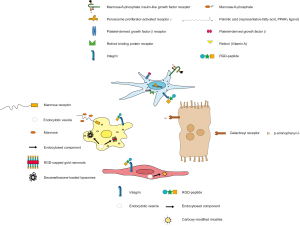
Influencing macrophages using nanomedicine
Immunomodulatory effects of nanoparticles on macrophages have been reported independently by different groups. We found that even nanoparticle surface chemistry alone appears to have the potential to modulate macrophage polarization (29) and nanoparticle-conjugated peptides were shown to induce macrophage activation (23,30). Such a “pre-activation” of macrophages may have tremendous consequences in disease conditions, as shown for RGD-peptide modified AuNRs, which significantly increased liver injury in a hepatitis model in mice (16) (Table 1). Other groups have shown that even unmodified silica-based nanoparticles (SiO2) may trigger hepatic injury by inducing a KC-based release of reactive oxygen species and proinflammatory cytokines like TNF which leads to hepatotoxicity in vitro and in vivo (28). Other groups even observed fibrogenesis upon repetitive injection of silica nanoparticles, with activated KC that play a key role in this process (27).
Earlier work attempted to deliver the broad antiinflammatory corticosteroid dexamethasone to KC by coupling it to mannosylated albumin [Dexa(5)-Man(10)-HAS] (67). A study of our group, based on in vitro studies with primary human macrophages, indicated that the liposomal encapsulation of dexamethasone may be a highly efficient tool for macrophage targeting and for modulating their inflammatory cytokine responses as well as their migratory properties (8). Interestingly, we found that dexamethasone-loaded liposomes were also efficient in vivo by ameliorating inflammatory liver diseases both in a model of acute hepatitis and in chronic carbon tetrachloride (CCl4)-based chronic toxic liver injury. These therapeutic effects coincided with a pronounced M2 activation profile of macrophages, but also with a significant reduction in the number of T cells in the liver (60) (Table 1).
As the macrophage-mediated release of proinflammatory cytokines like TNF is deleterious in the progression of many liver diseases, anti-TNF antibody treatment (such as infliximab) has been explored in acute alcoholic hepatitis. However, this broad and systemic inhibition of TNF was harmful in a clinical trial with patients, because it increased the rate of bacterial infections (68). As a promising novel strategy, it was proposed to inhibit TNF using small interfering RNA (siRNA) delivered via mannose-modified trimethyl chitosan-cysteine (MTC) conjugated nanoparticles that are internalized mostly by macrophages, based on a macrophage-specific delivery route via the mannose receptor (55). The system was shown to be more efficient than comparable systems for siRNA delivery and it offers the possibility for oral delivery, which could be very advantageous for the translation into clinical applications. This approach prevented inflammation-induced liver damage and lethality of mice in an experimental model of acute lipopolysaccharide/D-galactosamine liver disease after oral administration (55) (Table 1).
Monocytes, macrophages, and specifically dendritic cells have an additional bridging function for the activation of adaptive immunity. They recognize and phagocytize pathogens, can process their antigens and present them via major histocompatibility complex II to cells of the adaptive immune system, specifically to helper T cells. Thus, nanomaterials could thereby indirectly also influence cells of the adaptive immune system, specifically T cells—if the material interacts with the antigen processing of the antigen presenting cells (APC). As an example, liver cancer progression might be altered, if a certain nanomaterial induces alternative activation of macrophages, which would suppress anti-tumoral immunity (69). Importantly, dendritic cell maturation was also found to be regulated by peptide-modified AuNP, but the consequences for antigen processing and T cell activation are currently obscure (23).
Thus, macrophages and other APC play a major role due to their unspecific nanoparticle uptake and possibly also for the recognition of associated antigens that are processed for presentation to adaptive immunity. Macrophages therefore not only represent attractive targets for nanomedicine in liver disease, but they also need to be considered as potential particle scavenging cells in any kind of parenteral nanoparticle administration.
Different strategies for targeting HSC using nanomedicine
Activated HSC are considered as key target cells for liver fibrosis therapy, because they are the dominant contributors to ECM production in experimental fibrosis (70). HSC express multiple different surface structures that could serve as targets for nanoparticles. The most prominent targeted molecule is probably the mannose-6-phosphate/insulin-like growth factor receptor (M6P/IGFII receptor), which is upregulated on activated HSC during liver fibrosis (71). It is involved in the activation of the latent transforming growth factor β (L-TGF-β), which regulates TGF-β, a profibrogenic cytokine that induces collagen production (72). Drug delivery to HSC can be realized by targeting the M6P/IGFII receptor using human albumin decorated with 28 M6P groups (termed M6P-HSA), a compound which is internalized by and therefore accumulates in HSC (73). Using M6P-HSA for liposome coating increased the liver uptake of the decorated liposomes by 2.6 fold and triggered an increased clearance of the coated liposomes from the circulating blood (56) (Table 1). It was shown that via this route, also DNA can be delivered to HSC, for example, inactivated hemagglutinating virus of Japan (HVJ) was loaded into M6P-HSA-modified liposomes and was successfully delivered to HSC (74). This nanocarrier system can potentially be used to transport antifibrotic drugs to activated HSC in the liver. Luk and colleagues have shown that M6P-HSA coupled to the surface of liposomes loaded with 18β-glycyrrhetinic acid, but not the equivalent dose of the drug, rapidly translocate to the liver of rats that underwent bile duct-ligation-based experimental liver fibrosis and the drug significantly attenuated fibrosis (57) (Table 1). It was shown that 18β-glycyrrhetinic acid acts via its inhibition of free fatty acid-induced lipotoxicity (75).
Another targetable structure in the nucleus of HSC are peroxisome proliferator-activated receptors (PPAR), nuclear hormone receptors that perform transcriptional control, which are involved in liver fibrogenesis (76). Most clearly, PPARγ promotes the transformation process of HSC to myofibroblasts with a decreased expression during their transdifferentiation. Rosiglitazone increases PPARγ expression and thereby inhibits HSC activation (77). M6P-HSA-based delivery of the PPARγ ligand rosiglitazone, which increased liver accumulation of the drug, inhibited HSC activation and attenuated fibrosis in a CCl4-based chronic liver injury model in rats (56).
The platelet-derived growth factor (PDGF) is probably one of the most critical factors that induces HSC proliferation in liver fibrosis. One of its two corresponding receptors, the PDGFRβ, is highly upregulated on activated HSC (78). There are promising studies demonstrating that a targeted sterically-stabilized liposome (SSL) equipped with a cyclic peptide termed “C*SRNLIDC*” (pPB) with high affinity for the PDGF-β receptor and loaded with IFN-γ, improved the anti-fibrotic effects of IFN-γ in TAA-based hepatic fibrosis in mice while at the same time reducing its side effects (58) (Table 1).
Another promising molecular structure to target HSC is the retinol binding protein (RBP) receptor expressed by HSC, which is responsible for the uptake and storage of retinol (vitamin A). Retinol-coupled liposomes loaded with siRNA against heat-shock protein 47, which acts as a collagen-specific chaperone, were shown to reverse fibrosis in different experimental mouse models such as bile duct ligation (BDL), diethylnitrosamine, and CCl4 (59) (Table 1).
Thus, there are many routes by which HSC can be targeted and possibly, a combination of different strategies might be helpful to inhibit or reverse fibrosis in a therapeutic setting in clinical practice.
A considerably different strategy is the delivery of anti-apoptotic compounds to specific liver cell types. Curcumin (diferuloylmethane) is an antiinflammatory plant extract that is generated from the rhizome of Curcuma longa (79). It has anti-fibrotic activity (80), however, drug efficacy suffers from the low solubility of Curcumin in water (81). Researchers have developed a nano-formulated polymer-based compound termed NanoCurcTM, which greatly enhanced the bioavailability and its intrahepatic concentration; in fact, this polymer-based compound significantly ameliorated CCl4-based fibrosis in mice. NanoCurcTM accumulates in multiple different cell types of the liver, namely hepatocytes and non-parenchymal cells, and it acts via inducing apoptosis of HSC, suppressing ECM protein production by HSC, and by increasing levels of hepatoprotective glutathione (9) (Table 1). Similarly, studies facilitating polymeric vesicles (polymerosomes, PM) equipped with surface RGD and oxymatrine (OM) (RGD-PM-OM) significantly reduced BDL-induced liver injury and fibrosis in rats (61) (Table 1), due to HSC targeting and killing.
Integrins are central regulators of fibrosis in many organs (82). Integrins mediate the interaction of HSC with their ECM molecules such as collagen and fibronectin. All integrins have a common motif in their ligands, the peptide sequence RGD (arginine-glycine-aspartic acid), which has been studied by many groups (83-85). Consequently, RGD can be used to target integrins and activated HSC. This strategy has been used successfully to deliver IFNγ-1b to HSC using SSLs that were surface-modified with cyclic RGD peptide and significantly reduced BDL-based liver fibrosis (62) (Table 1).
However, RGD might not be the ultimate targeting sequence as also macrophages or LSEC might be affected by RGD-coupled particles.
Manipulation of LSEC using nanomedicine
Similar to the macrophages, LSEC possess phagocytic activities. It was shown that in vitro, other endothelial cells such as human umbilical vein endothelial cells (HUVEC) exhibit remarkable phagocytic capabilities in short-term experiments of one hour (23). However, in vivo, the same particles were not located in LSEC 24 hours after intravenous administration, but in the hepatic macrophages (16). In 2013, a patent has been published which is based on the induction of tolerance in liver due to influencing regulatory T cells by LSEC-directed carboxy-modified micelles. The micelles translocate to LSEC and KC, and have T cell epitopes associated with their surface, can deliver antigen, and induce the generation of regulatory T cells, which suppress autoimmunity. Thereby, these complex nanoparticles are intended to induce tolerance against autoantigens, thereby ameliorating autoimmune diseases (63) (Table 1). It represents a future perspective in the development of multifunctional immunomodulatory nanoparticles.
Other groups have shown that fluorescent viral particles and virus protein-coated gold particles demonstrated a preferential uptake of the viral substrates into LSEC in vitro and in vivo. At later stages, the viruses leave the LSEC and enter the hepatocytes, in which they replicate (86). Therefore, using viral pathways of cell entry by masking nanoparticles may be useful for a cell specific targeting in liver or other organs, which should be used to decorate particles intended for the delivery of drugs to liver cells.
Hepatocyte targeting nanomedicine
Hepatocyte targeting is challenging, because these cells lack specific surface receptors, which would enable specific binding of antibodies or peptides. Mimicking natural nanocomplexes such as lipoproteins appears to hold promising potential for hepatocyte-directed nanomedicine. Recently, apolipoprotein mimicking nanocarriers, termed lipopeptide nanoparticles (LPNP), were shown to efficiently target and to deliver siRNA to hepatocytes in vivo, with orders of magnitude more efficiently than to non-parenchymal cells. The uptake was shown to occur via dynamin-dependent macropinocytosis (10). Future studies will reveal whether this strategy for administration will be efficient in treating liver diseases.
Sodium arsenite (NaAsO2) is a natural contaminant of groundwater, but also occurs due to excessive human mining activities. It promotes hepatic fibrosis via inducing oxidative stress in hepatocytes (87). The antioxidant polyphenolic flavonoid quercetin (QC) was shown to reduce arsenite-induced fibrosis via liposomes or polylactide nanocapsules. Interestingly, polylactide nanocapsules were more efficient in delivering QC to liver compared to liposomes (88). A more specific targeting of hepatocytes using liposomes was facilitated by galactosylating liposomal carriers (89). Galactosylation of liposomes with p-aminophenyl δ-D-galactopyranoside, which binds to the galactosyl receptor of hepatocytes, significantly reduced arsenite-induced fibrosis (64) (Table 1).
Conclusions and perspectives
Currently, the most promising applications for cell targeting in liver diseases work with the first generation of nanoparticles, predominantly with liposomes and micelles. Advantages of liposome-based nanosystems include their low toxicity, as known from already approved applications (such as liposomal amphotericin B as an antifungal drug), and their cost-efficient production. With more advanced nanoparticle formulations, the exact cellular subtypes targeted may be more complex. We found KC and iMΦ, two major macrophage subsets in the liver, to be fundamentally different in their uptake of nanoparticles; for instance, upon intravenous administration in mice, the iMΦ had internalized 30 times more AuNRs than the Kupffer cells (16). However, in case of the polymer-based nanoparticles, rather the KC were scavenging the particles but not the iMΦ (unpublished observations), and in case of liposomes, the uptake by both cell types was similar (60). Such differences may have important functional consequences, as the role of the subtypes in liver diseases is different and to some extent non-redundant (38).
More recent advances in the field of nanoparticle development have not been extensively tested in preclinical models of liver diseases until today. The solid lipid nanoparticles (SLN) evolved as an advancement of the comparatively soft liposomes, based on solid components that are stable both at room and body temperature, and thereby, enable a prolonged drug release (as drug solubility in solid liquid is lower) (24). SLN can have a lipid core and be functionalized and stabilized with polymers that reduce the unspecific cellular uptake by phagocytic cells like macrophages and endothelial cells (20).
The class of nanostructured lipid carriers (NLC) is comprised of a lipid matrix that protects an inner load and such kind of particles are in use in cosmetics and pharmacological products and were intended to replace liposomes, emulsions or polymeric nanoparticles (90). As a consequence of the large lipid content, these particles offer the advantages that they contain less water than for example liposomes, and have a higher drug loading capacity, or longer physical stability, based on the properties of their components. Future particles for liver cell targeting might be further optimized by using nanomedicine from the second generation of nanomedicine such as SLN and NLC.
Taken together, nanomedicine has evolved as an exciting and promising field of research for the treatment of liver diseases. It can be envisioned that tailoring of nanoparticles will allow to specifically target crucial cell types of the liver and deliver potent antiinflammatory or anti-fibrotic drugs with low systemic toxicity. Nevertheless, the most efficient nanoparticles and targeting approach as well as schedule of treatment remain to be defined in the future.
Acknowledgements
The authors thank all members of the Tacke lab, the Department of Medicine III and collaborating groups for helpful discussions. This work was supported by the German Research Foundation (DFG Ta434/2-1, DFG SFB/TRR57), by the Interdisciplinary Center for Clinical Research (IZKF) Aachen and by the START program of the Medical Faculty of the RWTH Aachen University (to MB).
Disclosure: The authors declare no conflict of interest.
References
- Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J 2008;411:1-18. [PubMed]
- Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology 2014;147:577-594. [PubMed]
- Giannitrapani L, Soresi M, Bondi ML, et al. Nanotechnology applications for the therapy of liver fibrosis. World J Gastroenterol 2014;20:7242-51. [PubMed]
- Pockros PJ, Jeffers L, Afdhal N, et al. Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-gamma1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology 2007;45:569-78. [PubMed]
- Tripathi D, Therapondos G, Lui HF, et al. Chronic administration of losartan, an angiotensin II receptor antagonist, is not effective in reducing portal pressure in patients with preascitic cirrhosis. Am J Gastroenterol 2004;99:390-4. [PubMed]
- Nelson DR, Tu Z, Soldevila-Pico C, et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology 2003;38:859-68. [PubMed]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-69. [PubMed]
- Bartneck M, Peters FM, Warzecha KT, et al. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomedicine 2014;10:1209-20. [PubMed]
- Bisht S, Khan MA, Bekhit M, et al. A polymeric nanoparticle formulation of curcumin (NanoCurc) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest 2011;91:1383-95. [PubMed]
- Dong Y, Love KT, Dorkin JR, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci U S A 2014;111:3955-60. [PubMed]
- Minati L, Antonini V, Torrengo S, et al. Sustained in vitro release and cell uptake of doxorubicin adsorbed onto gold nanoparticles and covered by a polyelectrolyte complex layer. Int J Pharm 2012;438:45-52. [PubMed]
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 2008;83:761-9. [PubMed]
- Nikoobakht B, Burda C, Braun M, et al. The quenching of CdSe quantum dots photoluminescence by gold nanoparticles in solution. Photochem Photobiol 2002;75:591-7. [PubMed]
- Chen J, Saeki F, Wiley BJ, et al. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett 2005;5:473-7. [PubMed]
- Krichevski O, Markovich G. Growth of colloidal gold nanostars and nanowires induced by palladium doping. Langmuir 2007;23:1496-9. [PubMed]
- Bartneck M, Ritz T, Keul HA, et al. Peptide-Functionalized Gold Nanorods Increase Liver Injury in Hepatitis. ACS Nano 2012;6:8767-77. [PubMed]
- Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165-70. [PubMed]
- Lammers T, Subr V, Ulbrich K, et al. HPMA-based polymer therapeutics improve the efficacy of surgery, of radiotherapy and of chemotherapy combinations. Nanomedicine (Lond) 2010;5:1501-23. [PubMed]
- Kümmerer K, Menz J, Schubert T, et al. Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 2011;82:1387-92. [PubMed]
- Müller RH, Maassen S, Weyhers H, et al. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J Drug Target 1996;4:161-70. [PubMed]
- Jain TK, Reddy MK, Morales MA, et al. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 2008;5:316-27. [PubMed]
- Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int 2006;26:1175-86. [PubMed]
- Bartneck M, Keul HA, Wambach M, et al. Effects of nanoparticle surface coupled peptides, functional endgroups and charge on intracellular distribution and functionality of human primary reticuloendothelial cells. Nanomedicine 2012;8:1282-92. [PubMed]
- Bartneck M, Keul HA, Zwadlo-Klarwasser G, et al. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett 2010;10:59-63. [PubMed]
- Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small 2007;3:1941-9. [PubMed]
- Shi H, Magaye R, Castranova V, et al. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 2013;10:15. [PubMed]
- Liu T, Li L, Fu C, et al. Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials 2012;33:2399-407. [PubMed]
- Zelman WN, McLaughlin CP. Product lines in a complex marketplace: matching organizational strategy to buyer behavior. Health Care Manage Rev 1990;15:9-14. [PubMed]
- Bartneck M, Keul HA, Singh S, et al. Rapid uptake of gold nanorods by primary human blood phagocytes and immunomodulatory effects of surface chemistry. ACS Nano 2010;4:3073-86. [PubMed]
- Bastús NG, Sánchez-Tilló E, Pujals S, et al. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano 2009;3:1335-44. [PubMed]
- Magami Y, Azuma T, Inokuchi H, et al. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver 2002;22:419-25. [PubMed]
- Macdonald RA. “Lifespan” of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med 1961;107:335-43. [PubMed]
- Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci 2005;10:3093-9. [PubMed]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 2001;21:311-35. [PubMed]
- Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol 2012;6:67-80. [PubMed]
- Pradere JP, Kluwe J, De Minicis S, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 2013;58:1461-73. [PubMed]
- Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol 2009;183:7623-9. [PubMed]
- Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014;60:1090-6. [PubMed]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996-1006. [PubMed]
- Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79-91. [PubMed]
- Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 2009;50:261-74. [PubMed]
- Sheth K, Bankey P. The liver as an immune organ. Curr Opin Crit Care care 2001;7:99-104.
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc 2002;50:2041-56. [PubMed]
- Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One 2010;5:e11049. [PubMed]
- Mullarky IK, Szaba FM, Berggren KN, et al. Tumor necrosis factor alpha and gamma interferon, but not hemorrhage or pathogen burden, dictate levels of protective fibrin deposition during infection. Infect Immun 2006;74:1181-8. [PubMed]
- Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40:274-88. [PubMed]
- Trindade MC, Lind M, Nakashima Y, et al. Interleukin-10 inhibits polymethylmethacrylate particle induced interleukin-6 and tumor necrosis factor-alpha release by human monocyte/macrophages in vitro. Biomaterials 2001;22:2067-73. [PubMed]
- Wisse E, De Zanger RB, Charels K, et al. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 1985;5:683-92. [PubMed]
- Knolle PA, Limmer A. Control of immune responses by savenger liver endothelial cells. Swiss Med Wkly 2003;133:501-6. [PubMed]
- Wu J, Meng Z, Jiang M, et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 2010;129:363-74. [PubMed]
- Dinarello CA. Proinflammatory cytokines. Chest 2000;118:503-8. [PubMed]
- Sørensen KK, McCourt P, Berg T, et al. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol 2012;303:R1217-30. [PubMed]
- Steffan AM, Gendrault JL, McCuskey RS, et al. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 1986;6:830-6. [PubMed]
- Lohse AW, Knolle PA, Bilo K, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 1996;110:1175-81. [PubMed]
- He C, Yin L, Tang C, et al. Multifunctional polymeric nanoparticles for oral delivery of TNF-alpha siRNA to macrophages. Biomaterials 2013;34:2843-54. [PubMed]
- Patel G, Kher G, Misra A. Preparation and evaluation of hepatic stellate cell selective, surface conjugated, peroxisome proliferator-activated receptor-gamma ligand loaded liposomes. J Drug Target 2012;20:155-65. [PubMed]
- Luk JM, Zhang QS, Lee NP, et al. Hepatic stellate cell-targeted delivery of M6P-HSA-glycyrrhetinic acid attenuates hepatic fibrogenesis in a bile duct ligation rat model. Liver Int 2007;27:548-57. [PubMed]
- Li F, Li QH, Wang JY, et al. Effects of interferon-gamma liposomes targeted to platelet-derived growth factor receptor-beta on hepatic fibrosis in rats. J Control Release 2012;159:261-70. [PubMed]
- Sato Y, Murase K, Kato J, et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 2008;26:431-42. [PubMed]
- Bartneck M, Scheyda KM, Warzecha KT, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials 2015;37C:367-82. [PubMed]
- Yang J, Hou Y, Ji G, et al. Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci 2014;52:180-90. [PubMed]
- Du SL, Pan H, Lu WY, et al. Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol Exp Ther 2007;322:560-8. [PubMed]
- Herkel J, Carambia A, Freund B, et al. Nanoparticle compositions for generation of regulatory T cells and treatment of autoimmune diseases and other chronic inflammatory conditions. Google Patents; 2013.
- Mandal AK, Das S, Basu MK, et al. Hepatoprotective activity of liposomal flavonoid against arsenite-induced liver fibrosis. J Pharmacol Exp Ther 2007;320:994-1001. [PubMed]
- Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 2008;48:920-30. [PubMed]
- Xie G, Wang X, Wang L, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012;142:918-927.e6.
- Melgert BN, Olinga P, Van Der Laan JM, et al. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology 2001;34:719-28. [PubMed]
- Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004;39:1390-7. [PubMed]
- Mossanen JC, Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology 2013;2:e26468. [PubMed]
- Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 2013;4:2823. [PubMed]
- de Bleser PJ, Jannes P, van Buul-Offers SC, et al. Insulinlike growth factor-II/mannose 6-phosphate receptor is expressed on CCl4-exposed rat fat-storing cells and facilitates activation of latent transforming growth factor-beta in cocultures with sinusoidal endothelial cells. Hepatology 1995;21:1429-37. [PubMed]
- Hayashi H, Sakai T. Biological Significance of Local TGF-β Activation in Liver Diseases. Front Physiol 2012;3:12. [PubMed]
- Beljaars L, Molema G, Weert B, et al. Albumin modified with mannose 6-phosphate: A potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology 1999;29:1486-93. [PubMed]
- Adrian JE, Kamps JA, Poelstra K, et al. Delivery of viral vectors to hepatic stellate cells in fibrotic livers using HVJ envelopes fused with targeted liposomes. J Drug Target 2007;15:75-82. [PubMed]
- Wu X, Zhang L, Gurley E, et al. Prevention of free fatty acid-induced hepatic lipotoxicity by 18beta-glycyrrhetinic acid through lysosomal and mitochondrial pathways. Hepatology 2008;47:1905-15. [PubMed]
- Zardi EM, Navarini L, Sambataro G, et al. Hepatic PPARs: their role in liver physiology, fibrosis and treatment. Curr Med Chem 2013;20:3370-96. [PubMed]
- Chen H, He YW, Liu WQ, et al. Rosiglitazone prevents murine hepatic fibrosis induced by Schistosoma japonicum. World J Gastroenterol 2008;14:2905-11. [PubMed]
- Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004;15:255-73. [PubMed]
- Notarbartolo M, Poma P, Perri D, et al. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett 2005;224:53-65. [PubMed]
- Yao QY, Xu BL, Wang JY, et al. Inhibition by curcumin of multiple sites of the transforming growth factor-beta1 signalling pathway ameliorates the progression of liver fibrosis induced by carbon tetrachloride in rats. BMC Complement Altern Med 2012;12:156. [PubMed]
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807-18. [PubMed]
- Henderson NC, Arnold TD, Katamura Y, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 2013;19:1617-24. [PubMed]
- Patsenker E, Stickel F. Role of integrins in fibrosing liver diseases. Am J Physiol Gastrointest Liver Physiol 2011;301:G425-34. [PubMed]
- Levine D, Rockey DC, Milner TA, et al. Expression of the integrin alpha8beta1 during pulmonary and hepatic fibrosis. Am J Pathol 2000;156:1927-35. [PubMed]
- Zhou X, Murphy FR, Gehdu N, et al. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem 2004;279:23996-4006. [PubMed]
- Breiner KM, Schaller H, Knolle PA. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology 2001;34:803-8. [PubMed]
- Bera AK, Rana T, Das S, et al. L-Ascorbate protects rat hepatocytes against sodium arsenite--induced cytotoxicity and oxidative damage. Hum Exp Toxicol 2010;29:103-11. [PubMed]
- Ghosh D, Ghosh S, Sarkar S, et al. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact 2010;186:61-71. [PubMed]
- Managit C, Kawakami S, Yamashita F, et al. Effect of galactose density on asialoglycoprotein receptor-mediated uptake of galactosylated liposomes. J Pharm Sci 2005;94:2266-75. [PubMed]
- Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 2002;54 Suppl 1:S131-55. [PubMed]


