Potential use of Doppler perfusion index in detection of occult liver metastases from colorectal cancer
Introduction
According to epidemiological research, colorectal cancer is the second most common cancer and the second most common cause of death from malignant disease in Europe, causing approximately 400,000 deaths annually worldwide (1-4). The main cause of death of patients with colorectal cancer is liver metastases (5). It is well known that approximately 25% of patients with colorectal cancer already have liver metastases, and another 25% of patients develop liver metastases during follow up, usually within the first 2 years after the diagnosis of the primary colorectal tumor (6). In rectal cancer, preoperative chemoradiotherapy (CRT) significantly reduces the rate of local recurrence (5.3% vs. 14.1%), but patients who were treated with preoperative CRT do not appear to benefit significantly in terms of their long-term prognosis (66.2% vs. 67.8%) (7). Also, improvements in surgical technique resulted in less local recurrence (8).
Currently there is no reliable method for detecting small, occult liver metastases. Oncologists use various prognostic factors in deciding on adjuvant treatment. A standard prognostic factor that is used routinely in selecting patients for adjuvant treatment is the Dukes classification of the primary colorectal cancer (9-11). The survival of patients with dukes C stage as well as one part of patients with dukes B stage can be improved by the application of adjuvant chemotherapy after potentially curative surgical resection (12,13). Adjuvant chemotherapy (5-fluorouracil with levamisole or 5-fluorouracil with folinic acid) leads to a 40% reduction in the rate of recurrence and metastases, and 33% reduction in mortality rates of patients with Dukes C colon cancer (14). Despite that, approximately one third of patients with Dukes C colon cancer will survive 5 years even without adjuvant chemotherapy. On the other hand, approximately one third of patients with Dukes B colon cancer will develop recurrent disease or metastases. However, today there is no clear recommendation for the application of adjuvant chemotherapy in patients with colorectal cancer stage Dukes B (9). Therefore, it is obvious that Dukes classification is insufficient for the selection of patients for the application of adjuvant chemotherapy after potentially curative resection for colorectal cancer (9).
Careful selection of patients is crucial to improve the results of chemotherapy by applying it only to patients with the greatest impact on survival and avoiding harmful effects of chemotherapy in patients with no risk off liver metastasis (15). Therefore, the detection of those patients with micrometastases that are not evident at the time of primary tumor treatment still represents a significant challenge (16).
Current morphological methods for diagnosing liver metastases from colorectal cancer obviously have the limitation in detecting small focal liver lesions less than a few millimeters in diameter (17).
Hepatic perfusion changes in patients with liver metastases
It has been known for a while now, that in patients with liver metastasis there is an alteration of blood flow through the liver (18). Alterations of the hepatic flow in tumors were initially observed using dynamic scintigraphy. In 1983 Parkin et al. (19) proved that when malignant tumors are present the arterial hepatic flow is elevated because tumors have a predominantly arterial vascularity. Parkin also proposed the use of the hepatic perfusion index (HPI), which is increased in patients with liver metastases.
Several papers consistently demonstrated that a relative blood flow through the common hepatic artery, expressed as a percentage of total hepatic blood flow is significantly increased in patients with hepatic metastases in comparison to patients without liver metastases (9,20,21).
For a long time it has been considered that the observed change of blood flow through the liver is exclusively the consequence of increased neovascularization within the metastases themselves. Clinical and experimental studies demonstrated that liver metastases from colorectal cancer establish their blood supply mostly through hepatic artery system (22-24).
Although some research demonstrated that liver micrometastases indeed do derive some of their blood flow through the portal system, portal vascularization of liver metastasis is generally considered insignificant in comparison to vascularization derived through hepatic artery, especially considering the fact that the with the growth of metastasis there is also an increase in arterial blood supply compared to a portal blood supply (23).
In spite of the well-known thinking that only metastasis greater than one millimeter in diameter receive their blood supply through newly formed blood vessels (22), it has been demonstrated that even metastasis with only half a millimeter have a defined vascularization derived predominantly through the system of hepatic artery (23,25). Hepatic neovascularization is a complex process during which the relative contribution of arterial and portal blood flow changes during the growth of liver metastases (24,25). In the earliest stage, liver metastasis depend on perfusion from adjacent issue, until they reach a diameter of approximately 150-200 µm (26). Further growth of the metastasis induces new vessel formation derived from those arterial and portal system. As liver metastasis increases to over two millimeters in size, arterial blood flow becomes dominant (27). Angiographic research demonstrated a great variability in the vascularization of liver metastasis. Some metastasis demonstrate minimal accumulation of contrast (hypovascularized metastases), while others are extremely well vascularized with a marked arterial supply of the entire liver lobe (24). These variations in blood supply of liver metastasis play a significant role in the choice and success of local therapeutic procedures such as locoregional arterial chemotherapy, dearterialization and embolisation.
Doppler perfusion index (DPI)
This well known change in liver perfusion in patients with liver metastasis was further investigated by Leen and colleagues using Doppler ultrasound. In a series of papers he demonstrated that a Doppler ultrasound is a simple, non-invasive and reliable method in detecting changes in liver perfusion, especially in patients with colorectal cancer liver metastases (9,28-30).
Some research demonstrated that DPI enables greater precision to determine the likelihood of the existence of occult metastases in the liver. The analysis of preoperative values of DPI on a sample of 120 patients with colorectal cancer confirmed the statistically significant predictive value of DPI the detection of liver metastasis. The sensitivity, specificity, positive and negative predictive values as well as the accuracy of determining DPI for identification of patients in whom liver metastasis will be diagnosed during follow up was found to be 95%, 69%, 73%, 94% and 81% (9).
Five-year follow-up of patients with colorectal cancer demonstrated that the blood flow redistribution through the liver is strongly correlated to the survival (9). In a prospective study, the 5-year follow-up of patients who underwent potentially curative resection of primary colorectal cancer found that patients with normal values of DPI (DPI <30%) had a 5-year rate of disease free survival of 89%, while the 5-year disease free survival was only 22% in patients with elevated DPI (DPI ≥30%). Overall survival of patients with normal values of DPI was as high as 91% and only 29% in patients with abnormally elevated DPI (9).
Measurement of blood flow through blood vessels by color Doppler is extremely dependent on a number of technical parameters, and even small errors in the measurement of the diameter or cross-sectional area of blood vessels or the angle at which the measurements were performed can produce large errors in the calculation of the blood flow through (Table 1) (31,32). Therefore, in most clinical studies where Doppler measurements were used to measure blood flow, mean values of several consecutive measurements of diameters or cross sectional areas of blood vessels were used to reduce the risk of errors in measurements (9). Analyzing the maximum speed of blood flow through the vessel in systole [peak systolic velocity (PSV)], the speed of blood flow at the end of diastole [end-diastolic velocity (EDV)] and calculating the resistance index (RI) can more precisely describe the hemodynamic status of the arteries than the calculation of blood flow volume, because the calculation of the above parameters does not necessitate to knowledge the cross-sectional surface of measured vessels.
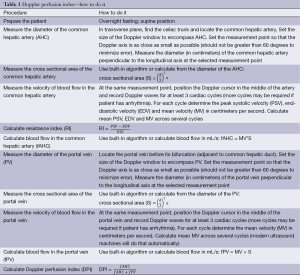
Full table
Despite the fact that ultrasonic Doppler technique is dependent on the examiner, technically challenging and often requires significant time to perform, it has confirmed good reproducibility and validity of the measurements among multiple examiners (33,34).
Moreover, satisfactory accuracy and reproducibility of measurements of the DPI of the liver was found when the measurements were performed by operators with no classical medical education and after only a few months of training in this specific area (18).
In spite of the standardization of measurements, values of blood flow often demonstrate wide dispersion (6). In order to maximize the extent of comparability and reduce the scattering of value, the flow can be expressed in relation to body surface area.
In histological examinations, the diameter of coronary arteries has been brought in correlation with age and body surface area off young healthy people (35). Body surface area is also used as a method of standardization of the cross sectional area of different arteries (36). Body surface area is routinely used in research of blood flow through different vessels as well as for liver haemodynamics (37). Therefore, utilizing body surface area and expressing the blood flow through different vessels relative to body surface area is a specially suitable to compare vascular parameters between groups with substantial morphological differences (38). Also, body surface area is used to achieve greater comparability of masses of different organs, such as liver, which mass, expressed relatively according to body surface area can be used in different researches (39). There are several formulas that enable the calculation of the body surface area, such as those recommended by Mosteller or DuBois and DuBois (Table 2) (40,43,44). The calculation of body surface area is a common procedure in many clinical and scientific branches of medicine (45), but surprisingly in most research this method of standardization was not used (6).

Full table
Another possible downfall of determining DPI with the purpose of detection of micrometastasis in the liver in patients with colorectal cancer is the factor that DPI is increased in patients with cirrhotic liver. However, hemodynamic examination of hepatic blood flow demonstrated that in patients with liver cirrhosis there is also an increase in liver congestion index, defined as the ratio of the cross sectional area of the portal vein and portal mean blood flow velocity (30).
However, not all authors were able to prove the clinical usefulness off DPI measurement in the detection of liver metastasis. In a clinical study conducted by Roumen et al. (46), 133 patients with different stages of colorectal cancer were examined. Reliable DPI measurements were not possible in 29 patients, mostly due to technical difficulties caused by the presence of air or other contrast media, obesity, scars or other reasons. In their study, they were unable to detect a single cut-off value that could reliably discriminate patients with liver metastases. It has to be noted that in this study no preselection of patients was performed and the focus was placed on the clinical usefulness of Doppler measurements in unselected population of patients. Apart from technical difficulties, Doppler perfusion measurements are characterized by high variability, which has been reported to be as high as 26% (46). Especially important it is the intraobserver variability that is not merely the result of the method or technique but rather a consequence of inherent subject variations.
As the Doppler measurements may well prove not to be useful in everyday practice, the underlying hypothesis of the existence of a humoral vasoactive substance responsible for hepatic perfusion changes sheds the new light on the clinical and preclinical research directed towards finding an easily and reliably measured systemic factor.
Possibility of a humoral vasoactive factor
Until now, the cause of this redistribution of hepatic blood flow is not clearly explained. According to one hypothesis, this phenomenon is caused by splanchnic vasoconstriction and a consequent reduction of portal blood flow with a simultaneous increase in common hepatic artery flow as a result of hemodynamic compensation (15). Some experimental and clinical research indeed demonstrated that in patients with liver metastases that are too small to be detected by conventional radiologic methods there is already an alteration in the blood flow through the liver that was shown to be highly sensitive in the detection of small, occult liver metastasis (9,18,20,47). This raises the question on the nature of these haemodynamic changes, since these small, occult metastases are unlikely to be the cause of sufficient neovascularization responsible for significant changes in hepatic perfusion (Table 3). It was therefore hypothesized that the primary cause of hepatic perfusion changes in patients with colorectal cancer liver metastases is a circulating vazoactive factor causing primarily splanchnic vasoconstriction and subsequent reduction in portal inflow to the liver.
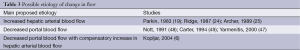
Full table
In order to prove the hypothesis of circulating vasoactive factor as a possible cause of liver hemodynamic changes in patients with colorectal cancer and liver metastasis, a group of researchers conducted a research on animal model (49). In this research, isolated intestinal loop of a healthy animal was perfused with blood from another animal with experimentally induced liver sarcoma (HSN sarcoma) (49).
The experiment showed that splanchnic vascular resistance in healthy animals was significantly greater during perfusion with blood from tumor bearing animals [91.6 (SE 21.5), vs. 51.7 (SE 7.41), P=0.036]. The results of this experiment suggest that observed hemodynamic changes are at least partly mediated by a circulating agent. Whether this circulating agent is produced by the tumor itself or is an endogenous agent remains unclear (49).
In another animal experiment (47), liver metastases were induced in 30 male Wistar rats by inoculating Walker 256 tumor subcutaneously. Hemodynamic changes were observed and correlated to the liver histology at the time of measurement. By measuring the flow through the hepatic artery and portal vein in this animal model of spontaneous liver metastases, Yarmenitis and colleagues have shown a statistically significant increase in blood flow through the common hepatic artery as early as the fourth day after implantation of the primary tumor, when histological examination of the liver demonstrated only single tumor cells or small clusters in the connective tissue of porta hepatis and periportal interlobular space (47).
DPI values were significantly increased as early as on the fourth day after implantation of the primary tumor, and did not significantly increase until the fifteenth day, when the histological examination showed metastatic tumors in the liver with the largest diameter of 2 mm. Also, the flow through the portal vein was reduced in animals with metastatic tumors in the liver on the fourth day, but the difference was not statistically significant. Therefore, a statistically significant increase in DPI and blood flow through the hepatic artery was observed only four days after implantation of tumor cells, when the histological analysis of the liver in these animals was not able to demonstrate any vascular component of either the hepatic artery or portal vein.
This finding is consistent with the hypothesis of the existence of a humoral vasoactive factor that can lead to hemodynamic changes in the liver in the earliest stages of the development of metastases, and that its effect may manifest both locally, in the liver, as well as in the splanchnic circulation away from metastases (49). Opposite to some other studies (48,49), in the experimental study conducted by Yarmenitis and associates, DPI changes were primarily attributed to a statistically significant increase in the flow through the hepatic artery and without significant changes in portal flow (47).
In the research conducted by Nott and coworkers (48), the blood flow through the portal vein was significantly reduced in animals with experimentally induced sarcoma of the liver (Walker carcinosarcoma). The results of this study indicate that overt tumor derived from the intraportal inoculation of Walker cells results in an increase in the HPI. The blood supply to the tumor was shown to be derived principally from the hepatic artery. However, hepatic arterial flow did not change in the presence of tumor and the alterations in the HPI were found to be secondary to a reduction in portal venous inflow. Moreover, the presence of overt hepatic tumor was associated with gross derangement of hepatic hemodynamic with a pronounced increase in intrahepatic arteriovenous shunting. It was concluded that hemodynamic changes accompanying the development of overt hepatic tumor are complex and must be taken into account when attempting to potentiate the distribution of cytotoxics to the tumor by regional administration or through manipulation of liver blood flow (48).
Other researchers also demonstrated significant reduction of portal flow in experimental models of liver metastasis, with no changes in arterial hepatic flow (50). An experimental and biomolecular research identified some systemic active factors that might, at least in theory, explain the redistribution of blood flow through the liver in patients with colorectal liver metastasis (51).
One possible causative agent might be endothelin-1 (15), a potent vasoconstrictor with significant influence on the portal blood flow that is regularly produced by the colorectal cancer. Peeters and coworkers measured the serum level of endothelin-1 in 68 patients with colorectal cancer and 20 healthy volunteers without malignant disease. The sera level of endothelin-1 was statistically significantly higher in patients with colorectal cancer compared to healthy participants. Further subgroup analysis was performed and patients were divided into three groups: those with primary colorectal cancer without metastasis, patients with colorectal cancer that developed metastasis during follow up and patients with colorectal cancer and synchronous liver metastasis. All three subgroups had higher concentrations of endothelin-1 compared to healthy participants. However, no statistically significant difference in preoperative concentration of endothelin-1 was found between healthy participants and patients with liver metastasis from previously resected colorectal cancer (15).
Animal models demonstrated that endothelin-1 results in increased blood pressure in the portal vein (52,53). Also, it has been determined that endothelin-1 is synthesized and released from several human epithelial carcinomas. Inagaki and coworkers demonstrated the existence of increased quantities of endothelin-1 in the tissue of primary colorectal carcinoma (54). Furthermore, histochemical methods demonstrated the presence of endothelin-1 in the cytoplasm of colorectal cancer cells metastatic to the liver, as well as in cytoplasm of adjacent myofibroblasts. These results indicate that endothelin-1 is not produced only in tumor cells but also in adjacent cells, thereby influencing the growth of the tumor (51). Unfortunately, survival analysis did not demonstrate prognostic value of pre-operative determination of the concentration of endothelin-1 in patients with colorectal cancer (15).
In one clinical study (6) results showed that the DPI successively increased in patients with colorectal cancer with no signs of liver metastases and in patients with liver metastases. There was a statistically significant difference in the blood flow through the portal vein between patients with colorectal cancer without signs of metastases in the liver and healthy control patients, but no statistically significant differences in the flow through the common hepatic artery (6). However, the flow through the common hepatic artery and portal vein in patients with primary colorectal cancer with no signs of liver metastases and those with liver metastases, as well as between patients with liver metastases and control subjects showed statistically significant differences in the absolute values of flow through common hepatic artery and portal vein as well as the values of the flow through the same blood vessels expressed relative to body surface area (6).
These results suggest that the early hemodynamic changes in patients with liver metastases are associated with a reduction of flow through the portal vein, while the increase in blood flow through the common hepatic artery is associated with the development of tumor neovascularization in larger metastases (6).
Furthermore, clinical research demonstrated no statistically significant differences in the blood flow through the superior mesenteric artery between patients with liver metastases and those without metastases, and the difference in RI was marginally statistically significant (6).
These findings are certainly at least partly influenced by the difficulties in measuring the cross-sectional area of blood vessels and blood flow in general, which is why the measurements of vascular index have the advantage.
The hypothesis of the existence of systematic acting, circulating humoral vasoactive factor in patients with liver metastases is further supported by the analysis results obtained by measuring the flow rate and RI of the superior mesenteric artery among the three groups of patients (patients with liver metastases, those with primary colorectal cancer and no detectable metastases and patients with no malignancy). If this hypothesis is correct, and if indeed a humoral vasoactive factors can be found in the bloodstream of patients with metastases in the liver, then its actions should be expressed also on remote vessels, outside of the liver.
Indeed, the EDV in the superior mesenteric artery was higher in healthy subjects compared to patients with colorectal cancer and no signs of liver metastases as well as compared to patients with liver metastases, while the difference in end-diastolic velocity in the superior mesenteric artery in patients with colorectal cancer without signs of metastases in the liver and patients with metastases was not statistically significant (6). Statistically significant differences of RI were found among healthy subjects, patients with colorectal cancer without signs of metastases in the liver and patients with metastases (6). These results clearly indicate that even in patients with colorectal cancer without clinical and radiological signs of metastases in the liver, there is a systemic alteration of blood flow, which is reflected in the reduction of the end-diastolic velocity in the superior mesenteric artery.
Conclusions
In the last three decades, many clinical and preclinical studies demonstrated that DPI measurements may be used to accurately diagnose and predict liver metastases from primary colorectal cancer. However, Doppler measurements have some serious limitations when applied to general population. Ultrasound is very operator-dependent, and requires skilled examiners. Also, many conditions may limit the use of Doppler ultrasound and ultrasound in general, such as the presence of air in digestive tract, cardiac arrhythmias (including rather common atrial fibrillation), vascular anomalies (e.g., the origin of right hepatic artery from superior mesenteric artery), obesity and other conditions (6,46). Therefore, in spite of the results from clinical studies, its value may be limited in everyday practice.
On the contrary, scientific research of the DPI in detection of liver metastases is of great importance, since current research speaks strongly for the presence of systemic vasoactive substance responsible for observed hemodynamic changes. Identification of such a systemic vasoactive substance may lead to the development of a simple and reproducible laboratory test that may reliably identify the presence of occult liver metastases and therefore increase the success of adjuvant chemotherapy through better selection of patients. Further research in this subject is therefore of great importance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Boyle P, Langman JS. ABC of colorectal cancer: Epidemiology. BMJ 2000;321:805-8. [PubMed]
- Jurisić I, Paradzik MT, Jurić D, et al. National program of colorectal carcinoma early detection in Brod-Posavina County (east Croatia). Coll Antropol 2013;37:1223-7. [PubMed]
- Milas J, Samardzić S, Miskulin M. Neoplasms (C00-D48) in Osijek-Baranja County from 2001 to 2006, Croatia. Coll Antropol 2013;37:1209-22. [PubMed]
- Samardzić S, Mihaljević S, Dmitrović B, et al. First six years of implementing colorectal cancer screening in the Osijek-Baranja County, Croatia--can we do better? Coll Antropol 2013;37:913-8. [PubMed]
- Doko M, Zovak M, Ledinsky M, et al. Safety of simultaneous resections of colorectal cancer and liver metastases. Coll Antropol 2000;24:381-90. [PubMed]
- Kopljar M, Brkljacic B, Doko M, et al. Nature of Doppler perfusion index changes in patients with colorectal cancer liver metastases. J Ultrasound Med 2004;23:1295-300. [PubMed]
- Boras Z, Kondza G, Sisljagić V, et al. Prognostic factors of local recurrence and survival after curative rectal cancer surgery: a single institution experience. Coll Antropol 2012;36:1355-61. [PubMed]
- Krebs B, Kozelj M, Potrc S. Rectal cancer treatment and survival--comparison of two 5-year time intervals. Coll Antropol 2012;36:419-23. [PubMed]
- Leen E, Goldberg JA, Angerson WJ, et al. Potential role of doppler perfusion index in selection of patients with colorectal cancer for adjuvant chemotherapy. Lancet 2000;355:34-7. [PubMed]
- Cunningham D, Findlay M. The chemotherapy of colon cancer can no longer be ignored. Eur J Cancer 1993;29A:2077-9. [PubMed]
- Bosman FT. Prognostic value of pathological characteristics of colorectal cancer. Eur J Cancer 1995;31A:1216-21. [PubMed]
- Haller DG. An overview of adjuvant therapy for colorectal cancer. Eur J Cancer 1995;31A:1255-63. [PubMed]
- van Triest B, van Groeningen CJ, Pinedo HM. Current chemotherapeutic possibilities in the treatment of colorectal cancer. Eur J Cancer 1995;31A:1193-7. [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995;122:321-6. [PubMed]
- Peeters CF, Thomas CM, Sweep FC, et al. Elevated serum endothelin-1 levels in patients with colorectal cancer; relevance for prognosis. Int J Biol Markers 2000;15:288-93. [PubMed]
- Fong Y. Doppler perfusion index in colorectal cancer. Lancet 2000;355:5-6. [PubMed]
- Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol 2005;23:8713-6. [PubMed]
- Leen E. The detection of occult liver metastases of colorectal carcinoma. J Hepatobiliary Pancreat Surg 1999;6:7-15. [PubMed]
- Parkin A, Robinson PJ, Baxter P. Liver perfusion scintigraphy. Method, normal range and laparotomy correlation in 100 patients. Nuclear Medicine Communications 1983;4:395-402.
- Leen E, Angerson WJ, Wotherspoon H, et al. Detection of colorectal liver metastases: comparison of laparotomy, CT, US, and Doppler perfusion index and evaluation of postoperative follow-up results. Radiology 1995;195:113-6. [PubMed]
- Leen E, Goldberg JA, Robertson J, et al. Early detection of occult colorectal hepatic metastases using duplex colour Doppler sonography. Br J Surg 1993;80:1249-51. [PubMed]
- Ackerman NB. The blood supply in liver metastases. In: Weiss L, Gilbert HA. eds. Liver Metastases. Boston: Hall; 1982,96-125.
- Lin G, Lunderquist A, Hägerstrand I, et al. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery 1984;96:517-26. [PubMed]
- Ridge JA, Bading JR, Gelbard AS, et al. Perfusion of colorectal hepatic metastases. Relative distribution of flow from the hepatic artery and portal vein. Cancer 1987;59:1547-53. [PubMed]
- Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg 1989;76:545-8. [PubMed]
- Strohmeyer T, Haugeberg G, Lierse W. Angioarchitecture and blood supply of micro- and macrometastases in human livers. An anatomic-pathological investigation using injection-techniques. J Hepatol 1987;4:181-9. [PubMed]
- Ackerman NB. Experimental studies on the role of the portal circulation in hepatic tumor vascularity. Cancer 1986;58:1653-7. [PubMed]
- Leen E, Goldberg JA, Robertson J, et al. The use of duplex sonography in the detection of colorectal hepatic metastases. Br J Cancer 1991;63:323-5. [PubMed]
- Robertson J, Leen E, Goldberg JA, et al. Flow measurement using duplex Doppler ultrasound: haemodynamic changes in patients with colorectal liver metastases. Clin Phys Physiol Meas 1992;13:299-310. [PubMed]
- Leen E, Goldberg JA, Anderson JR, et al. Hepatic perfusion changes in patients with liver metastases: comparison with those patients with cirrhosis. Gut 1993;34:554-7. [PubMed]
- Breyer B. Medicinski ultrazvuk - uvod u fiziku i tehniku. Zagreb: Školska knjiga; 1989. Available online: http://www.skolskaknjiga.hr
- Burns PN. Physics and instrumentation: doppler. In: Goldberg BB. eds. Textbook of abdominal ultrasound. Baltimore: Williams & Wilkins; 1993, 24-9. Available online: http://www.lww.com
- Oppo K, Leen E, Angerson WJ, et al. Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology 1998;208:453-7. [PubMed]
- Krüger S, Strobel D, Wehler M, et al. Hepatic Doppler perfusion index--a sensitive screening method for detecting liver metastases? Ultraschall Med 2000;21:206-9. [PubMed]
- Litovsky SH, Farb A, Burke AP, et al. Effect of age, race, body surface area, heart weight and atherosclerosis on coronary artery dimensions in young males. Atherosclerosis 1996;123:243-50. [PubMed]
- Huonker M, Schmid A, Schmidt-Trucksass A, et al. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol (1985) 2003;95:685-91. [PubMed]
- Bombelli L, Genitoni V, Biasi S, et al. Liver hemodynamic flow balance by image-directed Doppler ultrasound evaluation in normal subjects. J Clin Ultrasound 1991;19:257-62. [PubMed]
- Zeppilli P, Vannicelli R, Santini C, et al. Echocardiographic size of conductance vessels in athletes and sedentary people. Int J Sports Med 1995;16:38-44. [PubMed]
- Kuo PC, Li K, Alfrey EJ, et al. Magnetic resonance imaging and hepatic hemodynamics: correlation with metabolic function in liver transplantation candidates. Surgery 1995;117:373-9. [PubMed]
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303-11; discussion 312-3. [PubMed]
- Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep 1970;54:225-35. [PubMed]
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 1978;93:62-6. [PubMed]
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. [PubMed]
- Wang Y, Moss J, Thisted R. Predictors of body surface area. J Clin Anesth 1992;4:4-10. [PubMed]
- Favier M, de Cazanove F, Saint-Martin F, et al. Preventing medication errors in antineoplastic therapy. Am J Hosp Pharm 1994;51:832-3. [PubMed]
- Roumen RM, Scheltinga MR, Slooter GD, et al. Doppler perfusion index fails to predict the presence of occult hepatic colorectal metastases. Eur J Surg Oncol 2005;31:521-7. [PubMed]
- Yarmenitis SD, Kalogeropoulou CP, Hatjikondi O, et al. An experimental approach of the Doppler perfusion index of the liver in detecting occult hepatic metastases: histological findings related to the hemodynamic measurements in Wistar rats. Eur Radiol 2000;10:417-24. [PubMed]
- Nott DM, Grime SJ, Yates J, et al. Changes in hepatic haemodynamics in rats with overt liver tumour. Br J Cancer 1991;64:1088-92. [PubMed]
- Carter R, Anderson JH, Cooke TG, et al. Splanchnic blood flow changes in the presence of hepatic tumour: evidence of a humoral mediator. Br J Cancer 1994;69:1025-6. [PubMed]
- Hemingway DM, Cooke TG, Grime SJ, et al. Changes in hepatic haemodynamics and hepatic perfusion index during the growth and development of hypovascular HSN sarcoma in rats. Br J Surg 1991;78:326-30. [PubMed]
- Shankar A, Loizidou M, Aliev G, et al. Raised endothelin 1 levels in patients with colorectal liver metastases. Br J Surg 1998;85:502-6. [PubMed]
- Okumura S, Takei Y, Kawano S, et al. Vasoactive effect of endothelin-1 on rat liver in vivo. Hepatology 1994;19:155-61. [PubMed]
- Dugdale PE, Miles KA. Hepatic metastases: the value of quantitative assessment of contrast enhancement on computed tomography. Eur J Radiol 1999;30:206-13. [PubMed]
- Inagaki H, Bishop AE, Eimoto T, et al. Autoradiographic localization of endothelin-1 binding sites in human colonic cancer tissue. J Pathol 1992;168:263-7. [PubMed]


