
Robotic-assisted versus open total pancreatectomy: a propensity score-matched study
Introduction
Total pancreatectomy (TP) is a technically challenging operation associated with increased perioperative morbidity and mortality (1,2), whereas the resulting endocrine and exocrine insufficiency has a significant impact on patients’ quality of life (3). Even though eligibility criteria for TP are strict, this approach is increasingly utilized for a wide range of pancreatic diseases that include multifocal pancreatic neuroendocrine tumors, main duct intraductal papillary mucinous neoplasms (MD-IPMN), pancreatic ductal adenocarcinoma (PDAC), chronic pancreatitis (CP) and even multifocal metastatic pancreatic tumors (4-6). The current optimization of postoperative complication management in pancreatic surgery and improved approaches in the management of postoperative pancreatic endocrine and exocrine insufficiency permit the adoption of TP as an option for the treatment of highly selected patients (7).
In recent years, the prevalence of minimally invasive techniques for pancreatic resections has been significantly increasing. Retrospective studies and recent randomized trials demonstrate that both laparoscopic and robotic-assisted pancreatic surgery is efficacious and safe (8-11). Regarding the latter, an increasing amount of data demonstrates the effectiveness and feasibility of robotic-assisted pancreatic surgery with perioperative and oncological outcomes, which is comparable to open approaches (12-14). However, data on the role of robotic-assisted total pancreatectomy (RTP) are limited; only small retrospective studies have provided an early insight into the potential of this novel approach (15-18). Our department of pancreatic surgery is a high-volume center. In the past 3 years, we have performed 1,000 pancreatectomies annually (year 2017: 1,002; year 2018: 1,089; year 2019: 1,077) including 300 annual robotic pancreatectomies (year 2017: 352; year 2018: 299; year 2019: 271). This study presents a series of patients who underwent RTP, demonstrates their perioperative and long-term outcomes, and compares the results to a cohort of patients who underwent open total pancreatectomy (OTP).
We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.19/rc).
Methods
Patient cohort and data collection
All patients who underwent TP from March 2015 to July 2019 in the Pancreatic Surgery Department of Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine were eligible for this retrospective study. The decision to offer a robotic or open approach for TP was decided on an individual basis by the surgical team by considering patient performance status, body habitus, and the status of previous complex abdominal surgeries in combination with patient preference. Patients who underwent completion pancreatectomy for tumor recurrence or as an emergency management of postoperative complications were excluded from the study. Furthermore, patients who underwent near-TP without duodenectomy for any indication or TP for acute necrotizing pancreatitis were also excluded from the cohort.
The prospectively maintained institutional database and electronic medical records were primarily utilized for the collection of clinical and demographic data, perioperative surgical outcomes, pathology results, and long-term patient follow-up. Patients were reviewed every 3 months through outpatient clinic appointments that consisted of a physical examination, laboratory tests for endocrine and exocrine insufficiency assessment, updated imaging, and a quality of life evaluation. Intermittent communications via phone were also conducted at shorter time intervals. The Clavien-Dindo classification system was utilized to assess postoperative complications (19).
All of the included patients had previously signed an informed consent and agreed to data collection. This study was approved by the institutional review board of Shanghai Ruijin Hospital (No. 2017-180). The study was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
SPSS 22.0 (IBM, Chicago, IL, USA) and the R statistical packages (The R Foundation; http://www.r-project.org; version 3.4.3) were utilized for statistical analysis and propensity score matching (PSM). Propensity scores were based on baseline characteristics including age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) status, vascular involvement, position and distribution of the lesion, involvement of adjacent organs, artery variation, and pathology types. The matching was performed in a 1:1 ratio, and a caliper width of 0.2 standard deviations (SDs) was specified. Continuous data were summarized as mean values and SDs or the median values, interquartile intervals and ranges (IQRs) were utilized. Student’s t-test or the Mann-Whitney U test was used for comparison of continuous variables, and the chi-square or Fisher’s exact test was used for analysis of categorical variables; categorical variables were expressed as numbers and percentages. A P value of <0.05 was considered statistically significant.
Operative technique for RTP
For all cases, the da Vinci Si Surgical System (Intuitive Surgical Inc. Sunnyvale, CA, USA) was utilized, and a 5-port trocar placement was set up as previously described (20-23) (Figure 1). Dissection was performed using harmonic shears, fenestrated bipolar forceps, and Cadiere forceps; the harmonic shears was switched to a needle holder for secure suture and reconstruction. Standard endoscopic staplers were utilized for transection of the jejunum and distal stomach.
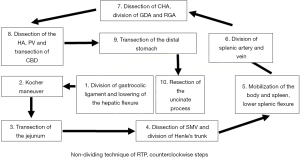
A traditional “dividing technique” was employed for solitary benign or borderline tumors of the pancreas with transection of the pancreatic parenchyma at the pancreatic neck and separation of the procedure into two steps: pancreaticoduodenectomy (PD) followed by distal pancreatectomy (DP). When this approach was applied for malignant lesions, assessment of the resection margin was done with a frozen section, and a subsequent TP was performed if the margin was positive and the remnant pancreas was not suitable for anastomosis. In patients with MD-IPMN or selected malignant lesions without vascular involvement, an en bloc resection of the pancreatic gland was performed (nondividing technique). For all RTPs, the dissection was initiated by dividing the gastrocolic ligament and continued in an antegrade counterclockwise fashion as demonstrated in Figure 2: an extended Kocher maneuver was initially performed for mobilization of the duodenum with identification of the vena cava followed by transection of the jejunal loop approximately 10 cm from the duodenojejunal flexure. Dissection around the SMV/PV/SV was then performed with division of the gastrocolic trunk when indicated. Further mobilization of the distal pancreas and spleen was followed by assessment, dissection, and division of the splenic vessels when a concurrent splenectomy was necessary. In patients where spleen preservation was planned, either the Kimura or Warshaw technique was utilized based on an individual basis (24,25). The next step included dissection of the hepatoduodenal ligament with identification of the common hepatic artery (CHA) and division of the gastroduodenal artery (GDA) and right gastric artery. The common bile duct was divided, and the dissection continued medially with transection of the distal stomach. The whole specimen was shifted towards the right side, and the last step involved resection of the uncinate process with division of the pancreatoduodenal venous branches and the inferior pancreaticoduodenal artery.
In patients with potential vascular involvement, thorough preoperative assessment of the reconstruction was performed, and an “artery-first” approach was always utilized (26-28). When a venous resection was deemed intraoperatively necessary, clamping with laparoscopic bulldog clamps was performed prior to the last step of tumor dissection from the PV/SMV confluence. Vascular reconstruction was performed based on the vein deficit, either as a primary closure or an end-to-end anastomosis, both with continuous 5-0 nonabsorbable sutures. Reconstruction of the choledochojejunostomy and gastrojejunostomy was conducted in a standardized single-layer continuous fashion, and a single abdominal drain was routinely placed. Drain removal occurred on postoperative day 5–7, based on the patient’s postoperative course, volume of drain fluid, and imaging when available. In all cases, patient-directed oral intake was followed postoperatively, most often with a liquid diet from postoperative day 3. Long-acting insulin was initiated after establishment of adequate oral intake with strict glucose surveillance and a subsequent dose adjustment. Pancreatic enzyme supplementation was also administered at the same time.
When a simultaneous islet-cell autotransplantation (IAT) was planned at the time of RTP, a “dividing technique” was employed with division of the pancreatic neck and performance of a DP as a first step. The distal pancreas and spleen were immediately resected after transection of the splenic vessels for optimal preservation of a viable islet cell population. A PD was then performed, while the distal pancreas was being cleaned and perfused. Prompt extraction of the PD specimen was performed after division of the GDA. Islet cell isolation was performed overnight at the laboratory, and cell pellets were injected into the portal vein (PV) under local anesthesia on postoperative day 1 using ultrasound and confirmed by digital subtraction angiography (DSA) in a hybrid operation room.
Results
Patient cohort
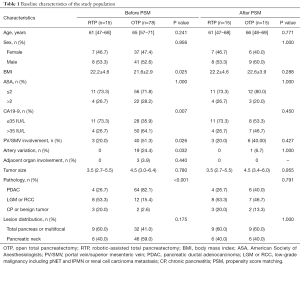
Overall, 117 patients were deemed eligible for inclusion within the studied time period (Figure 3). Twenty-four patients were excluded (21%) because they underwent completion pancreatectomy (n=14), duodenum-preserving TP (n=3), or acute necrotizing pancreatitis was the indication for surgery (n=7). Of the remaining 93 patients, 15 underwent an RTP (16%) and 78 an OTP (84%). A comparison of the two groups demonstrated that patients who underwent OTP had significantly lower BMI compared to the ones in the RTP group (P=0.025, Table 1). Additionally, OTP patients were found to more often have vascular involvement by the tumor (P=0.026) and aberrant arterial anatomy (P=0.032). Pancreatic adenocarcinoma was the main indication for surgery in the OTP group (82.1%) when compared that of the RTP group where benign lesions were encountered more often (73.3%, P<0.001). No differences were identified in tumor location distribution. After PSM, no differences were identified in patient characteristics between the two cohorts (Table 1).
Full table
Intraoperative and postoperative outcomes
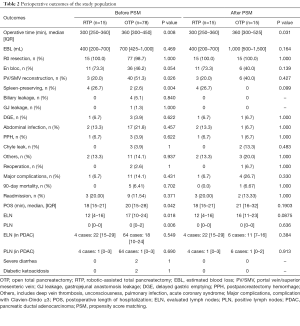
Intraoperative outcomes were also comparable, but specific key differences were identified (Table 2). Operative time was significantly lower in the RTP group (300 vs. 360 min, P=0.008), and the difference remained significant after PSM (P=0.031). Additionally, on initial analysis, patients in the RTP cohort were more likely to undergo spleen-preserving resection (P=0.004) and less likely to undergo venous resection and reconstruction (P=0.026). Furthermore, the median postoperative length of stay was lower in the RTP group (18 vs. 20 days, P=0.042); in terms of pathology, lymph node resection was more effective in the open approach (17 vs. 12 harvested nodes, P=0.018). All aforementioned differences were effectively eliminated after PSM.
Full table
One patient in the RTP group underwent a simultaneous IAT. The warm ischemia time was 10 min, and the isolation time was 150 min. The total islet yield was 200,000 islet equivalents (IEQ), which corresponded to a total dose of 4,000 IEQ per kilogram of body weight.
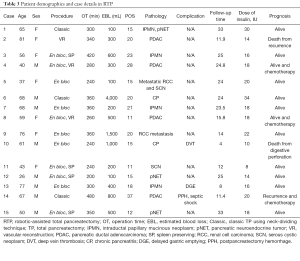
Postoperative complication rates were similar between the two groups. The major 30-day morbidity (Clavien-Dindo > IIIa) was 6.7% (n=1) and 14.1% (n=11) in the RTP and OTP group, respectively (P=0.431). No statistical difference was seen after PSM. A detailed presentation of clinical data and outcomes of RTP patients is available in Table 3.
Full table
Long-term follow-up and oncological outcomes
The median follow-up for all patients was 15 (IQR, 8–24) months. Two patients with severe diarrhea and two patients with diabetic ketoacidosis in the OTP group were readmitted after 3 months. None of the patients in the RTP group presented with severe symptoms from the resulting postoperative endocrine or exocrine insufficiency. In the RTP group, two patients with PDAC presented with disease recurrence at 11 months after the operation, and one of them succumbed to the disease immediately after recurrence. One additional patient died due to a perforation of the digestive tract at 4 months. In the OTP group (after PSM), two patients died because of liver failure caused by a PV thrombosis after PV/SMV reconstruction at 1 and 7 months. One patient had a recurrence 10 months after the surgery and died 3 months later.
Discussion
The main reason for limited utilization of TP is the significant postoperative long-term morbidity (3,29) due to the development of brittle diabetes (Type 3c) and the establishment of exocrine insufficiency that occasionally outweighs the benefits of the operation (regarding patient quality of life or even survival). However, improvements in surgical techniques and optimization of perioperative care of patients who undergo TP have allowed acceptable morbidity and mortality and favorable long-term outcomes in these patients (2,5).
The implementation of minimally invasive pancreatic surgery has paved the road for a similar approach in TP (10,30). The first case series was performed with a laparoscopic approach (11,31-34), but robotic-assisted TP reports were soon published (15-17,35,36). Initially, surgeons had to overcome the combination of a learning curve in robotic surgery and the surgical challenges of TP, which explains why most patients in the first series had a diagnosis of CP or benign pancreatic lesions (15,17,36). Nevertheless, the extra degree of freedom with the robotic approach allows more precise dissection of vascular pedicles and accurate manipulation of the tissues (37). Some researchers believe that the more complicated the surgery is, the more robotic surgery should be applied (38). So far, there have been seven publications on RTP ranging from one to eleven patients (Table 4). The most comprehensive series is from Boggi et al. in 2015 (15), which demonstrated for the first time the feasibility of a robotic approach in TP. However, in this report, we focus on the description of the en bloc resection of the pancreas. Furthermore, we compared patient outcomes with a propensity-matched cohort of patients who underwent OTP.
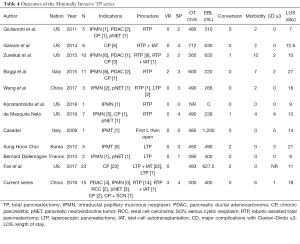
Full table
In the RTP group, there was an equal distribution of patients with benign (CP, cystic lesions or low-grade neuroendocrine tumors) and malignant disease [pancreatic adenocarcinoma or renal cell carcinoma (RCC) metastases] (53% and 47%, respectively). As expected, the proportion of patients with PDAC in the OTP group was higher, which explains the statistical difference in CA19-9 levels, BMI and vascular involvement. All patients with PDAC necessitating a TP were classified as locally advanced or borderline resectable; surgical resection in these patients was associated with longer operative time, increased estimated blood loss (EBL), occurrence of vascular reconstruction, and increased incidence of postoperative complications (2). To reduce bias from confounding variables, we utilized the concept of PSM to optimally compare the two groups: after PSM, the two cohorts had a similar distribution of malignant disease diagnosis and subsequent vascular reconstruction.
Furthermore, the operative time in the RTP cohort was significantly lower compared to that in the OTP cohort. These results contradict previous reports that have demonstrated longer operative times for robotic resections (15,38,39). However, recent studies have showed that the operative time in robotic pancreatic surgery can be reduced after a variable learning curve of 20–80 cases (15,20,37,40,41). Our group managed to significantly decrease the operative time by utilizing a systematic modular resection pattern and by standardizing individual roles within the team (42). Previous accumulation of significant experience in robotic PD and DP allowed familiarization with a more complex approach in TP; therefore, our team passed the learning curve for robotic pancreatic resections by performing more than 300 robotic pancreaticoduodenectomies before initiating RTP.
Initially, the “dividing” technique was utilized with a combination of RPD followed by RDP. Yet, since more patients with malignant tumors were offered a robotic resection, we proceeded with a “nondividing” approach. The rationale was to minimize potential tumor dissemination caused by the transection of the pancreatic neck. Interestingly, most patients in the RTP cohort underwent an en bloc resection (73%) compared to 46% in the OTP cohort. The advantage of the robotic approach allows a more intricate application of the en bloc resection and the ability to clearly visualize the operative field. However, a limited operative surface and width of vision during the robotic procedure necessitated standardization of the operative steps to achieve safe and efficient manipulation. Therefore, an antegrade approach was applied for the en bloc RTP in a counterclockwise fashion. A standardized procedure allowed for optimal results: unlike other series (36), our docking of the DaVinci Si system was completed with a 5-port placement, similar to other robotic resections and robotic enucleation of our series with constant instruments and a fixed team (20-23); a 6th or 7th trocar was rarely placed after the beginning of the operation. When difficult exposure or uncontrollable bleeding was intraoperatively encountered, an additional 5-mm trocar was placed between the optical and No. 2 arm for better exposure and use of suction. One of the most challenging aspects of robotic surgery is achieving adequate communication and cooperation between the lead surgeon at the console and the scrubbed assistant, which is a main disadvantage compared to the laparoscopic approach.
In the RTP cohort, three patients with PDAC underwent a vein resection, and in two cases, it was performed when the “nondividing” en bloc technique was applied. Vein resection in pancreatic surgery has significantly increased and is mainly due to the establishment of neoadjuvant treatment (43). Previous reports have demonstrated the feasibility of vein resection in robotic pancreatic surgery with acceptable morbidity and mortality (44); however, the experience in RTP is very limited (27,45). In this study, all three patients underwent a segmental vein resection and reconstruction with an end-to-end anastomosis without the use of vein graft or patch. We believe that there is still a debate regarding the role of arterial resections in pancreatic surgery (46), more so in the setting of minimally invasive TP. Yet, it is an interesting field to explore since reports in open pancreatic surgery are promising (47). Spleen preservation was also achieved in 50% of patients with benign diagnoses who underwent RTP. Previous studies have demonstrated increased spleen preservation rates in patients who underwent robotic compared to laparoscopic DP (48,49), and our results on RTP show similar outcomes. Furthermore, no patients in the RTP group were converted to an open procedure. A recent study showed that conversion rates in minimally invasive pancreatic surgery are improving and appear to be significantly lower in robotic cases (50). It appears that precision in instrument movement and tissue manipulation achieved with the robot is crucial in that aspect.
A borderline increased postoperative stay (POS) was also identified in the RTP group; however, the median POS of all patients is significantly higher compared to a series from Europe and the United States (11,36). In western countries, increased healthcare costs and a developed network of patient care in the community facilitate earlier patient discharge; yet in China, patients remain in the hospital until fully recovered. This difference in hospitalization strategy may account for the increased POS in this study (51). Additionally, the RTP and OTP groups had comparable 30-day morbidity and 90-day mortality. In TP, there are no pancreatic fistula-associated complications with the absence of pancreatic anastomosis. However, the challenge of postoperative management remains in terms of endocrine and exocrine function regulation. The establishment of an apancreatic state and the consequent intestinal malabsorption and brittle diabetes have a significant impact on a patient’s quality of life. Especially regarding diabetes, the high frequency of alternating hypoglycemic and hyperglycemic events is not tolerable by many patients, and therefore, case selection is critical (3,29). One of the surgical modalities used to address postoperative endocrine insufficiency is IAT. In this study, we report the first successful RTP with IAT performed in China. This is a previously described procedure with multiple surgical variations (11,16) and increasing utilization (52). The potential advantage of the robotic approach is late blood supply preservation and optimization of the isolated islet cell population.
There are several limitations in this study. First, it is a retrospective case series and therefore subject to selection bias. Additionally, even though our team has significant experience in robotic pancreatic resections, the authors acknowledge that the results of the first 15 RTP procedures are somehow biased by a procedure-specific learning curve. This is also evident from the fact that most patients with PDAC were assigned to undergo an OTP. Furthermore, the RTP cohort is relatively small in comparison, even after PSM, which could explain in part the absence of significant differences between the two groups. Additionally, there is significant heterogeneity regarding the indications for TP and the patient’s background. Lastly, the follow-up time for the studied patients was relatively limited, and long-term outcomes of RTP will be further evaluated. However, this study demonstrates the feasibility of robotic-assisted TP in a selected cohort of patients when performed in high-volume centers with extended robotic experience and adds more information to the small pool of existing data indicating the necessity for prospective studies.
Conclusions
In this retrospective study, the application of RTP in a selected cohort of patients appears to be safe and feasible in the management of both benign and malignant pancreatic lesions. A “nondividing” en bloc technique in a counterclockwise fashion allows for optimal surgical results with comparable mortality and morbidity compared to an open approach. A standardized surgical protocol can decrease operative time without affecting patient outcomes. Further multicenter prospective studies are necessary to better identify candidate patients for RTP.
Acknowledgments
The authors would like to thank all the participating patients and their families as well as the investigators, research nurses, study coordinators, and operation staff.
Funding: This study is sponsored by the Guangci Outstanding Youth Training Program (GCQN-2017-B06) and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2019QNB26).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.19/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.19/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All of the included patients had previously signed an informed consent and agreed to data collection. This study was approved by the institutional review board of Shanghai Ruijin Hospital (No. 2017-180).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhayani NH, Miller JL, Ortenzi G, et al. Perioperative outcomes of pancreaticoduodenectomy compared to total pancreatectomy for neoplasia. J Gastrointest Surg 2014;18:549-54. [Crossref] [PubMed]
- Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg 2009;250:282-7. [Crossref] [PubMed]
- Stoop TF, Ateeb Z, Ghorbani P, et al. Impact of endocrine and exocrine insufficiency on quality of life after total pancreatectomy. Ann Surg Oncol 2020;27:587-96. [Crossref] [PubMed]
- Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg 2007;246:966-74; discussion 974-5. [Crossref] [PubMed]
- Hartwig W, Gluth A, Hinz U, et al. Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg 2015;261:537-46. [Crossref] [PubMed]
- Bahra M, Neuhaus P. Pancreas: is there still a role for total pancreatectomy? Nat Rev Gastroenterol Hepatol 2010;7:72-4. [Crossref] [PubMed]
- Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg 2016;33:335-42. [Crossref] [PubMed]
- Eguia E, Kuo PC, Sweigert PJ, et al. The laparoscopic approach to pancreatoduodenectomy is cost neutral in very high-volume centers. Surgery 2019;166:1027-32. [Crossref] [PubMed]
- Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443-50. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- Fan CJ, Hirose K, Walsh CM, et al. Laparoscopic total pancreatectomy with islet autotransplantation and intraoperative islet separation as a treatment for patients with chronic pancreatitis. JAMA Surg 2017;152:550-6. [Crossref] [PubMed]
- Girgis MD, Zenati MS, King JC, et al. Oncologic outcomes after robotic pancreatic resections are not inferior to open surgery. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- van Hilst J, Strating EA, de Rooij T, et al. Costs and quality of life in a randomized trial comparing minimally invasive and open distal pancreatectomy (LEOPARD trial). Br J Surg 2019;106:910-21. [Crossref] [PubMed]
- Raoof M, Nota CLMA, Melstrom LG, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: Analysis of the National Cancer Database. J Surg Oncol 2018;118:651-6. [Crossref] [PubMed]
- Boggi U, Palladino S, Massimetti G, et al. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc 2015;29:1425-32. [Crossref] [PubMed]
- Galvani CA, Rodriguez Rilo H, Samamé J, et al. Fully robotic-assisted technique for total pancreatectomy with an autologous islet transplant in chronic pancreatitis patients: results of a first series. J Am Coll Surg 2014;218:e73-8. [Crossref] [PubMed]
- Giulianotti PC, Addeo P, Buchs NC, et al. Early experience with robotic total pancreatectomy. Pancreas 2011;40:311-3. [Crossref] [PubMed]
- de Mesquita Neto JWB, Macedo FI, Liu Y, et al. Fully robotic total pancreatectomy: technical aspects and outcomes. J Robot Surg 2019;13:77-82. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 2015;29:3698-711. [Crossref] [PubMed]
- Chen S, Zhan Q, Jin JB, et al. Robot-assisted laparoscopic versus open middle pancreatectomy: short-term results of a randomized controlled trial. Surg Endosc 2017;31:962-71. [Crossref] [PubMed]
- Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18. [Crossref] [PubMed]
- Jin JB, Qin K, Li H, et al. Robotic enucleation for benign or borderline tumours of the pancreas: a retrospective analysis and comparison from a High-Volume Centre in Asia. World J Surg 2016;40:3009-20. [Crossref] [PubMed]
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg 1988;123:550-3. [Crossref] [PubMed]
- Kimura W, Inoue T, Futakawa N, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery 1996;120:885-90. [Crossref] [PubMed]
- Ironside N, Barreto SG, Loveday B, et al. Meta-analysis of an artery-first approach versus standard pancreatoduodenectomy on perioperative outcomes and survival. Br J Surg 2018;105:628-36. [Crossref] [PubMed]
- Cai Y, Gao P, Li Y, et al. Laparoscopic pancreaticoduodenectomy with major venous resection and reconstruction: anterior superior mesenteric artery first approach. Surg Endosc 2018;32:4209-15. [Crossref] [PubMed]
- Weitz J, Rahbari N, Koch M, et al. The "artery first" approach for resection of pancreatic head cancer. J Am Coll Surg 2010;210:e1-4. [Crossref] [PubMed]
- Scholten L, Latenstein AEJ, van Eijck C, et al. Outcome and long-term quality of life after total pancreatectomy (PANORAMA): a nationwide cohort study. Surgery 2019;166:1017-26. [Crossref] [PubMed]
- Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 2020;271:54-66. [Crossref] [PubMed]
- Wang X, Li Y, Cai Y, et al. Laparoscopic total pancreatectomy: case report and literature review. Medicine (Baltimore) 2017;96:e5869. [Crossref] [PubMed]
- Chapman BC, Paniccia A, Ryan C, et al. Laparoscopic spleen-preserving total pancreatectomy for a main-duct intraductal papillary mucinous neoplasm. Ann Surg Oncol 2017;24:560. [Crossref] [PubMed]
- Choi SH, Hwang HK, Kang CM, et al. Pylorus- and spleen-preserving total pancreatoduodenectomy with resection of both whole splenic vessels: feasibility and laparoscopic application to intraductal papillary mucin-producing tumors of the pancreas. Surg Endosc 2012;26:2072-7. [Crossref] [PubMed]
- Dallemagne B, de Oliveira AT, Lacerda CF, et al. Full laparoscopic total pancreatectomy with and without spleen and pylorus preservation: a feasibility report. J Hepatobiliary Pancreat Sci 2013;20:647-53. [Crossref] [PubMed]
- Gruessner RW, Cercone R, Galvani C, et al. Results of open and robot-assisted pancreatectomies with autologous islet transplantations: treating chronic pancreatitis and preventing surgically induced diabetes. Transplant Proc 2014;46:1978-9. [Crossref] [PubMed]
- Zureikat AH, Nguyen T, Boone BA, et al. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc 2015;29:176-83. [Crossref] [PubMed]
- Liu R, Zhang T, Zhao ZM, et al. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc 2017;31:2380-6. [Crossref] [PubMed]
- Coratti A, Annecchiarico M. Robot-assisted pancreatic surgery. Br J Surg 2014;101:593-4. [Crossref] [PubMed]
- Zenoni SA, Arnoletti JP, de la Fuente SG. Recent developments in surgery: minimally invasive approaches for patients requiring pancreaticoduodenectomy. JAMA Surg 2013;148:1154-7. [Crossref] [PubMed]
- Zhang T, Zhao ZM, Gao YX, et al. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc 2019;33:2927-33. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Shi Y, Wang W, Qiu W, et al. Learning curve from 450 cases of robot-assisted pancreaticoduocectomy in a high-volume pancreatic center: optimization of operative procedure and a retrospective study. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Giovinazzo F, Turri G, Katz MH, et al. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179-91. [Crossref] [PubMed]
- Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. [Crossref] [PubMed]
- Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg 2019;270:e139-41. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
- Strijker M, van Santvoort HC, Besselink MG, et al. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB (Oxford) 2013;15:1-10. [Crossref] [PubMed]
- Klompmaker S, de Rooij T, Koerkamp BG, et al. International validation of reduced major morbidity after minimally invasive distal pancreatectomy compared with open pancreatectomy. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Liu W, Yuan S, Wei F, et al. Inappropriate hospital days of a tertiary hospital in Shanghai, China. Int J Qual Health Care 2017;29:699-704. [Crossref] [PubMed]
- Chinnakotla S, Beilman GJ, Dunn TB, et al. Factors predicting outcomes after a total pancreatectomy and islet autotransplantation lessons learned from over 500 cases. Ann Surg 2015;262:610-22. [Crossref] [PubMed]




