
Liver transplantation: would it be the best and last chance of cure for hepatocellular carcinoma with major venous invasion?
Introduction
Hepatocellular carcinoma (HCC) is the 5th most common cancer and 3rd most common cause of cancer related death in the world (1). This disease leads to significant burden to the healthcare system in developed countries such as United Stated due to the observed growing incidence and HCC-related death (2). Given the high propensity of portal vein invasion, 10–20% of the HCC presents have concomitant portal vein tumour thrombus (PVTT). According to the latest update of Barcelona Clinic Liver Cancer (BCLC) staging, HCC with portal vein tumour thrombosis (PVTT) is classified as Grade C advanced HCC (3), meaning that patients of BCLC Grade C can only receive systemic therapy even they have relatively preserved liver function, and the survival is exceedingly limited (4,5).
HCC patients with PVTT are treated more aggressively in many Asian countries. According to the Hong Kong Liver Cancer (HKLC) staging system, HCC patients (solitary tumor and the size is less than 5cm) and with intra-hepatic (i.e., left or right portal vein) PVTT are classified as Stage IIb/IIIa, in which resection can still be offered as a curative treatment (6). Although the long-term survival of HCC patients with PVTT is worse than the PVTT free counterparts, 3-year survival of up to 20% had been constantly reported in many Asian centres. In comparison to other treatment modalities, the survival outcomes after hepatectomy is still better than trans-arterial chemo-embolization (7,8), trans-arterial radio-embolization (9), external radiotherapy (10), and systemic treatments (11,12).
Liver transplantation (LT) is regarded as the best treatment for HCC. The 5-year overall survival of over 80% is frequently reported by established LT centres (13-16). Since major vascular invasion is considered as an absolute contra-indication for this procedure, data concerning the efficacy of LT in the context of HCC with PVTT treatment is very limited. A recent study suggested that LT was feasible for HCC with segmental PVTT but not for lobar PVTT (17). However, the survival benefit of LT in comparison liver resection (LR) in this context remains uncertain. The aim of this study was to address this issue and to evaluate the oncological feasibility of LT for HCC with PVTT. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.09/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective analysis of anonymous clinical data and, according to institutional guidelines, did not require approval by the institutional research ethics board. All patients gave informed consent to surgery as well as use of their anonymous clinical data for research purposes.
Patient background
From January 2000 to December 2016, the clinic-pathological and follow-up data for all patients who received either LT or LR for HCC were reviewed. In the LR group, patients with positive resection margin was excluded from data analysis. This was a retrospective cohort of patients with HCC related major venous thrombosis treated in Queen Mary Hospital, The University of Hong Kong from 2000 to 2016. Patients who received either LR or LT as a treatment with curative intent were included. Paediatric patients, patients with final pathology showing cholangiocarcinoma or mixed cholangio-HCC were excluded from the study. All LTs in this series were performed in Author’s centre and no prisoner’s organ was used for transplantation in our centre. Technical details of hepatectomy (18) and LT (19) had been described in previous publications. In short, patients who are eligible for hepatectomy if there is absence of extrahepatic disease, adequate liver function (i.e., indocyanine green retention less than 18% in 15 minutes and the ratio of future liver remnant to estimated standard liver volume more than 30%) and significant comorbidity that preclude major hepatectomy under general anaesthesia. Patients with pathological evidence of PVTT will not receive adjuvant treatment if the resection margin is negative of HCC. Concerning the protocol of LT for HCC patients, Milan criteria (20) was adopted for patient selection for DDLT before 2002. After 2002, University of California at San Francisco (UCSF) criteria was adopted for patient selection (21). Details concerning MELD bonus scoring system, bridging therapy, and immunosuppressive regimen were reported in previous publications (22). However, evidence of major vascular invasion (i.e., portal vein or hepatic vein) on pre-transplant structural imaging represent an absolute contra-indication to proceed. In case of PVTT in explant pathology, m-TOR inhibitor will be started with minimization of calcineurin inhibitor in order to reduce the chance of recurrence. Adjuvant or systemic therapy will only be given in case of the detection of recurrence.
Statistical analyses
Demographic data, preoperative biochemistry, pathological findings and operative outcomes were retrieved from prospectively maintained database. Classification of PVTT followed Liver Cancer Study Group of Japan (LCSGJ) (23). Multi-variate analysis with Cox-regression model was used to determine factors associated with post-transplant survival of the patients. Prior to survival comparison between two groups, univariate analysis was performed to look for presence of heterogeneity. Propensity score matching was performed to reduce bias secondary to the presence of these heterogeneity using the nearest neighbour method. Survivals of patients were analysed using Kaplan-Meier curves and compared with Log-rank test. P value less than 0.05 were considered statistically significant.
Results
Characteristics of study population
From 2000 to 2016, HCC with tumour invasion to the portal vein invasion was confirmed pathologically in 88 patients. Majority (94.3%) of the patients had tumour invasion to the second order branch of the portal vein (Vp2). In patients who received LT, all PVTT were incidental finding in the DDLT group, while 4 out of the 10 LDLT recipient had known PVTT before the operation. The median age at operation was 55 [22–78] years old and male was the predominant gender in the population. Within the whole study population, 88.6% and 4.5% were hepatitis B and hepatitis C carrier respectively. The median AFP level and tumour size was 1,694 [2–1,112,000] ng/L and 8.9 [1.4–21.5] cm respectively. The median follow-up duration was 16 months.
Univariate, multi-variate and propensity score matching
After univariate analysis, size of tumour (P=0.016) and LT (P=0.007) were found to be associated with post-operative survival. After multivariate analysis, only LT was the only independent factor associated with overall survival (P=0.01, OR 0.329, 95% CI: 0.142–0.766). The 3-year overall and disease-free survival of patients in the LT and resection was 69.2% vs. 25.1% (P=0.007) and 64% vs. 10.4% (P<0.001) respectively.
Comparisons between patients who received resection (n=75) and transplantation (n=13) were therefore made. Characteristic of these two population was shown in Table 1. A number of heterogeneities were observed between these two groups, namely pre-operative AFP (P=0.017), platelet count, total bilirubin, MELD score, tumour size, and type of venous invasion (P<0.001). Among these factors, those which were known to be associated with tumour biology were used for propensity score matching. There was no significant heterogeneity between resection and transplantation in all factors associated with worse tumour biology, namely AFP level, tumour size and number, microvascular invasion, tumour differentiation, and type of venous invasion after matching (Table 1).
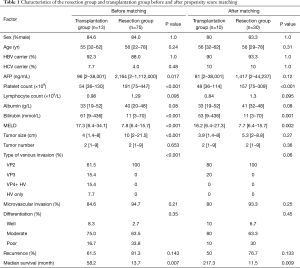
Full table
Survival outcomes in propensity score matched (PSM) population and subgroup analysis
There were 10 (7 DDLT and 3 LDLT) and 30 patients in the transplantation and resection group respectively after matching with their characteristics shown in Table 1. HCC recurrence was found in 50% and 76.7% of the patients in transplantation and resection group respectively (P=0.133). Patients in the transplantation group had a significantly better disease-free survival (median 62 vs. 5.7 months, P<0.001) (Figure 1). Superior overall survival was demonstrated in the transplantation group, with median survival of 217 vs. 12.3 months (P=0.009) (Figure 2). Survival analysis was repeated after excluding patients with pre-operative AFP over 500 ng/mL. A trend of superior overall survival was still observed in the transplantation group (Figure 3).

Discussion
Survival analyses in the matched population demonstrated that, patients in the LT group had significantly better disease-free and overall survival when compared to the resection group counterparts (62 and 217 vs. 5.7 and 12.3 months respectively). These superior oncological outcomes suggested that LT could be a viable treatment option in selected HCC patients with PVTT.
According to the BCLC staging, which was adopted by many liver cancer centres in the west, presence of PVTT represents an advance disease stage (stage C), as such, hepatectomy is no longer a treatment option. The expected survival of this group of patients is around 3 months. Hepatectomy with portal thrombectomy or en bloc thrombus resection technique had been proven safe and feasible for this group of patients. Despite the aggressiveness of the procedure, the reported median survival after surgery was still limited, ranging from only 8 to 36 (3-year overall survival of 0–49%) months from most series (18,24-29). The efficacy of hepatectomy as a curative treatment modality remains suboptimal.
Concerning LT, presence of PVTT is considered as an absolute contraindication judging from the high recurrence rate (30,31). There were few studies reporting the survival outcomes of LT for HCC patients with PVTT and most of them were from the Korean groups (17,32,33). The reported 5-yr overall survival was ranging from 50.3–63.6%. With these encouraging results, some surgeons advocated that PVTT should no longer be a contraindication for LT (32).
In the regions of organ shortage like many Asian countries (34), deceased liver graft is not allocated to HCC patients with PVTT in view of the anticipated worse outcomes comparing with HCC patients without PVTT. However, this principle of utility does not apply to LDLT in which the liver graft is a dedicated gift from a loved one. In fact, conventional criteria such as Milan Criteria and UCSF Criteria were introduced in more than 2 decades ago, there are many series reporting very good long term survival even transplanting HCC beyond these standard criteria. The message was clear that size and number of HCC represent only a part of the tumour biology, other factors such as alpha fetoprotein level, PIVKA-II level, nuclear differentiation, presence of microvascular and macrovascular invasion should all be put into the calculating formula. In a study by Choi et al., 24 patients with MVI were compared with 27 patients with segmental type PVTT (i.e., VP2). There was no statistical significant difference identified between two groups (3-year overall survival 69.7% vs. 60.3%, P=0.48) (17). This result suggested that HCC with PVTT should be seen as an adverse factor rather than an absolute contraindication for LT.
The best way to tell whether LT is a better treatment option than resection is to perform a randomized controlled trial, yet, this study design is practically not possible. Direct comparison between LT and resection is bound to bias due to obvious heterogeneity between these two populations. A propensity score matching analysis is probably next best methodology. In our study, because of the limited patient number, complete matching for all factors deemed not possible. Therefore, only factors known to be related to tumour biology were statistically matched (i.e., AFP, tumour size, type of PVTT). Despite a significantly better pre-operative liver function (i.e., MELD score, albumin level and platelet count) in the resection group population (better liver function is known to associate with better post-operative survival), the oncological outcomes were still inferior to that of the LT group. This implied a significant protective effective using LT as a treatment for HCC patient with PVTT.
It was observed that, despite statistically insignificant, the median AFP level in the resection group was still much higher than the LT group (1,417 vs. 87 ng/mL, P=0.12). The superiority of LT might be confounded by a lower pre-operative AFP level. Therefore, a subgroup survival analysis was performed for patients with AFP <500 ng/mL as this is a common cut-off for patient stratification used by many studies. Superiority of LT over resection was apparent from the wide separation of the corresponding Kaplan-Meier curves. An insignificant P value is likely accounted by type II error.
There were limitations concerning this study. The retrospective study design unavoidably subjected this study to selection and recall bias, use of propensity score matching helped to alleviate this weakness to a certain extent. In addition, small study population reduces the power of this study, yet this weakness is common in performing study on rare disease conditions (i.e., LT for HCC with PVTT). A multi-centre study would help to improve these weaknesses and better define the role of LT in the context of PVTT.
Conclusions
HCC with PVTT of Vp2/Vp3 type should be considered a relative contra-indication for LT in view of the potentially better oncological outcomes when compared to hepatectomy.
Acknowledgments
This article is accepted to be presented in Oral Session of APHPBA 2019 at Seoul, Korea.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.09/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.09/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.09/coif). Dr. ACYC and Dr. CML serve as unpaid editorial board members of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was a retrospective analysis of anonymous clinical data and, according to institutional guidelines, did not require approval by the institutional research ethics board. All patients gave informed consent to surgery as well as use of their anonymous clinical data for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol 2002;35:S72-8. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Schöniger-Hekele M, Müller C, Kutilek M, et al. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut 2001;48:103-9. [Crossref] [PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. [Crossref] [PubMed]
- Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413-20. [Crossref] [PubMed]
- Kim JH, Yoon HK, Kim SY, et al. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther 2009;29:1291-8. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Rim CH, Yang DS, Park YJ, et al. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol 2012;42:721-9. [Crossref] [PubMed]
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 2012;48:1452-65. [Crossref] [PubMed]
- Concejero A, Chen CL, Wang CC, et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation 2008;85:398-406. [Crossref] [PubMed]
- Takada Y, Uemoto S. Liver transplantation for hepatocellular carcinoma: the Kyoto experience. J Hepatobiliary Pancreat Sci 2010;17:527-32. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Wong TCL, Ng KKC, Fung JYY, et al. Long-Term Survival Outcome Between Living Donor and Deceased Donor Liver Transplant for Hepatocellular Carcinoma: Intention-to-Treat and Propensity Score Matching Analyses. Ann Surg Oncol 2019;26:1454-62. [Crossref] [PubMed]
- Choi HJ, Kim DG, Na GH, et al. The clinical outcomes of patients with portal vein tumor thrombi after living donor liver transplantation. Liver Transpl 2017;23:1023-31. [Crossref] [PubMed]
- Chok KS, Cheung TT, Chan SC, et al. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg 2014;38:490-6. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78-86. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Sharr WW, Chan SC, Lo CM. Section 3. Current status of downstaging of hepatocellular carcinoma before liver transplantation. Transplantation 2014;97:S10-7. [Crossref] [PubMed]
- Kudo M, Kitano M, Sakurai T, et al. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis 2015;33:765-70. [Crossref] [PubMed]
- Roayaie S, Jibara G, Taouli B, et al. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol 2013;20:3754-60. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]
- Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery 2012;152:809-20. [Crossref] [PubMed]
- Wei XB, Xu J, Li N, et al. The role of three-dimensional imaging in optimizing diagnosis, classification and surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. HPB (Oxford) 2016;18:287-95. [Crossref] [PubMed]
- Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol 2016;22:160-7. [Crossref] [PubMed]
- Gondolesi GE, Roayaie S, Munoz L, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg 2004;239:142-9. [Crossref] [PubMed]
- Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg 2004;239:150-9. [Crossref] [PubMed]
- Lee KW, Suh SW, Choi Y, et al. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl 2017;23:19-27. [Crossref] [PubMed]
- Han DH, Joo DJ, Kim MS, et al. Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis after Concurrent Chemoradiation Therapy. Yonsei Med J 2016;57:1276-81. [Crossref] [PubMed]
- Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol 2013;10:746-51. [Crossref] [PubMed]




