Hilar cholangiocarcinoma: diagnosis, treatment options, and management
Hilar cholangiocarcinoma (HC) was first described by Altemeier and Klatskin approximately 50 years ago and comprise over 60% of all cholangiocarcinomas (1-3). It is a complex and aggressive disease with a poor prognosis. We provide an evidence-based review of HC with a particular emphasis on the approach to management of this challenging disease. An electronic database search, including PubMed/MEDLINE, was performed using the terms hilar cholangiocarcinoma and Klatskin’s tumor. Additionally, a MESH database search was performed under the heading “Bile Duct Neoplasms” in combination with the aforementioned terms and Boolean operators AND or OR. Criteria for inclusion included English-language articles using human subjects (Figure 1).

Incidence and epidemiology
Cholangiocarcinoma accounts for less than 2% of all human malignancies but is the second most common primary liver tumor (4,5). Although rare in Western countries, it is more commonly seen in Asia with incidences as high as 113 per 100,000 men and 50 per 100,000 women (6). It is categorized as intrahepatic (ICC) or extrahepatic (ECC) according to the International Classification of Diseases for Oncology. There are approximately 3,000 ECC cases annually in the United States alone (5,7,8). ICC incidence has increased over the last 20 years while ECC has remained constant (9,10). However, this may be attributed to HC misclassification in the International Classification of Disease for Oncology system (8).
A variety of risk factors have been associated with HC including advanced age, male gender, cirrhosis, inflammatory bowel disease, and chronic pancreatitis (7). Parasitic liver disease (i.e., biliary ascariasis, liver flukes, and liver schistosomiasis) is an established HC risk factor along with biliary tract stone disease (4,7). The most well established risk factor for HC is primary sclerosing cholangitis (PSC). The lifetime incidence of cholangiocarcinoma (CC) in PSC patients is 6% to 36% with most patients presenting within 2.5 years of their PSC diagnosis (6,7,11). Notably, PSC involves both intra and extra hepatic bile ducts and PSC related CC is an equal risk factor for both ICC and ECC (12).
Presentation and diagnostic evaluation
HC generally presents in the 6th decade of life (13-16). Patients commonly present with jaundice, abdominal pain and weight loss (14-16). Fatigue, pruritus, nausea, dark urine and clay colored stools are also often seen (16). Biliary stones, inflammatory bowel disease, PSC, viral hepatitis are commonly encountered co-morbidities (16,17).
Over 80% of proximal biliary obstructions are secondary to HC (18-20). The remaining 15-20% are caused by benign strictures secondary to inflammatory disease, sclerosing cholangitis, stone disease, and gallbladder cancer invading the hepatoduodenal ligament (19,21-24). Both benign and malignant biliary strictures present with similar clinical features (19-21). Moreover, bilirubin and serum tumor marker levels do not reliably distinguish malignant and benign biliary strictures (21,23). Preoperative identification of benign biliary strictures remains uncertain therefore resection remains most appropriate (19-21,23).
Serum tumor markers, specifically carcinoembryonic antigen (CEA) and CA19-9, are used for the diagnosis, treatment, and monitoring of HC with 89% sensitivity and 86% specificity when combined with other diagnostic modalities (25). Additionally, tumor marker levels are associated with tumor stage. Tumors with higher levels at presentation are more likely to be unresectable, predicting a worse overall survival (25-27).
Imaging
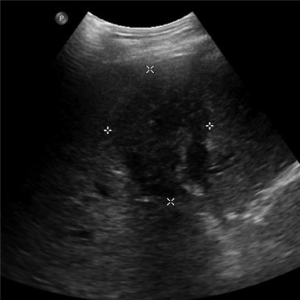
Less than one half of HC are resectable (28,29). Accurate radiological staging of these lesions is difficult secondary to the complexity of the hilar region, proximity to major vessels, and small tumor sizes (30). Computed topography (CT), magnetic resonance imaging (MRI), and ultrasound (US) are used to characterize suspicious biliary lesions. Initial radiographic assessment is usually transabdominal US due to its low cost and accessibility. It is sensitive for detecting biliary duct dilatation, but less sensitive in localizing the exact site of obstruction (31,32). Typically, any HC mass appears hypoechoic relative to surrounding liver parenchyma (Figure 2). US is unable to accurately determine the type of obstruction or extent of tumor involvement (33). In addition, US has relative poor sensitivity in identifying lymph node, liver, and peritoneal metastases and therefore further imaging modalities are generally needed (22,34).
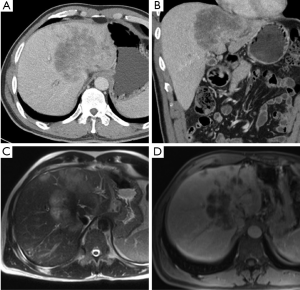
CT accurately predicts HC resectability in 60-90% of cases and is the most frequently used imaging modality to assess biliary tumor resectability (35-38). CT can help differentiate between benign and malignant strictures as well as depict the level of biliary obstruction (Figure 3). Arterial and portovenous phase CT can assist in delineating vascular invasion of the corresponding hepatic hilar structures (28,39). Thinly sliced (2-5 mm) multidetector CT (MDCT) correlates well (greater than 90%) with local tumor extension when compared with operative and pathological findings (36). In one systematic review of HC imaging techniques, CT was the most well studied imaging modality and demonstrated an acceptable accuracy (>80%) in assessing ductal, portal vein and hepatic artery involvement although it was unable to accurately assess lymph node involvement (29). Moreover, peritoneal metastases are generally underestimated (28,37). Therefore, despite reported high sensitivity and specificity for HC with CT imaging, metastatic involvement and spread to contiguous organs and vascular structures remains a possibility at the time of surgery even if undetected on CT.
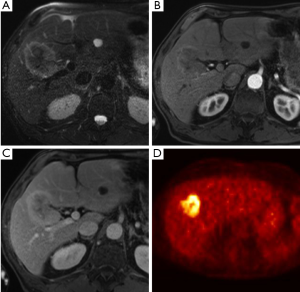
MRI has increasingly gained favor in assessing biliary tumors. HC appears as a hypointense signal on T1 weighted images and high signal intensity of T2 imaging (Figure 4). The tumor generally appears hypovascular in relation to adjacent hepatic parenchyma and may be characterized by irregular thickening of the bile duct wall with upstream dilation of intrahepatic bile ducts (Figure 5) (40). The combination of MRI with MRCP is about 80% accurate in predicting HC resectability (41-43). However, in a comparison of MRI combined with MRCP versus MDCT with direct cholangiography, Park and colleagues demonstrated no difference between the two groups in assessing HC resectability (44). Currently, the combination of MRCP and CT is favored over direct cholangiography. Unfortunately MRCP does not allow for invasive procedures such as biopsy, biliary drainage and stent insertion therefore direct cholangiography is still necessary in these instances.
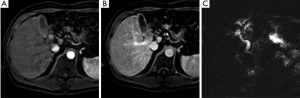
The role of PET/CT in HC remains less clear. PET/CT has a reported specificity of over 80% to detect lymph node and distant metastases, but it seemingly does not have much utility in assessing local resectability (45-47). Case studies utilizing PET/CTs are limited and more studies are needed to further evaluate the benefit of this imaging modality in HC. Currently, PET may be useful when assessing for metastatic disease but has no clear role in helping to evaluate issues of local resectability.
Direct cholangiography
Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC) assess local ductal extent of the tumor while allowing for therapeutic biliary drainage. The use of preoperative biliary drainage (PBD) in HC remains controversial. In Liu and colleagues systematic review comparing PBD with no PBD in resectable patients, the authors failed to note a benefit from PBD, although the lack of uniformity in the literature was a limitation of the analysis (48). Multiple retrospective reviews have shown that PBD in jaundiced HC patients decreases postoperative complications although no improvement in mortality or survival has been reported (14,49-52). Moreover multiple studies have shown that hepatic resection in jaundiced patients is associated with higher mortality and morbidity due to increased rates of liver failure and that PBD prior to hepatic resection can increase resectability (53-56). Kennedy and colleagues demonstrated improved perioperative outcomes with PBD in patients with future liver remnant (FLR) volume of less than 30% (57). Although randomized studies are needed to better address the potential benefits of PBD in HC, the literature indicates that PBD of FLR should be done routinely in jaundiced HC patients undergoing hepatic resection (58).
Both ERCP and PTC have similar sensitivity (75-85%) and specificity (70-75%) with regard to their ability to attain a tissue diagnosis; however, it is important to note that a negative biopsy cannot be considered definitive and an underlying HC should always be suspected in the right clinical setting regardless of the biopsy results (59-63). Direct comparisons have demonstrated less procedure related complications with PTC; however, PTC catheter tract recurrence may be seen in 2-5% of patients (64,65). Currently both approaches provide acceptable outcomes and the choice of approach is generally institution dependent.
Intraductal ultrasonography has acceptable sensitivity when compared with histology based staging, although this technique is also operator dependent and its accuracy can vary (38,66,67). Limited case series have demonstrated the feasibility of endoscopic US fine needle aspiration (FNA) to biopsy HC and regional lymph nodes although more studies are needed to understand the role of this technique in HC (68-71). Transperitoneal FNA of HC is associated with a higher rate of peritoneal metastases and should generally be avoided especially when curative resection is being considered (72).
MRCP has demonstrated similar efficacies to PTC and ERCP in identifying anatomic extension of tumors (31,60,73). Biliary staging is best assessed using MRCP while vascular and distant metastatic staging may be better assessed with either MDCT or contrast enhanced MRI (31). Preoperative biliary procedures such as stenting and percutaneous drainage induce biliary wall inflammation and create artifacts which hinders imaging interpretation. Therefore it is important for staging and assessment of resectability that cross-sectional imaging be obtained prior to biliary interventions whenever possible (32,33,41).
Staging systems
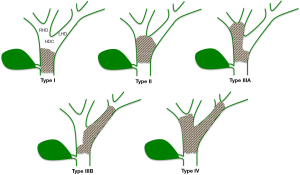
Various HC staging systems are currently used. The Bismuth-Corlette system provides preoperative assessment of local tumor spread and is used to determine the extent of resection (Figure 6) (74,75). It does not, however, provide information on vascular encasement or metastatic disease and is of limited prognostic value (7,76). The Memorial Sloan Kettering Cancer Center (MSKCC) classification details local tumor extent while also assessing portal vein involvement and hepatic lobar atrophy (Table 1). Similarly to the Bismuth-Corlette system, the MSKCC classification for HC does not account for metastatic or nodal disease and is most appropriate for categorizing local resectability (77-79).

Full table
The American Joint Committee on Cancer (AJCC) is the most commonly used staging system for CC (80). In the 7th edition of the AJCC staging system, extrahepatic CC was given its own independent staging that is further subdivided into perihilar and distal bile duct tumors. Unlike the Bismuth-Corlette and MSKCC staging systems, the AJCC system accounts for vascular encasement (both portal vein and hepatic artery), nodal involvement and distant metastases. It is mainly utilized as a postoperative staging system and has minimal utility in assessing resectability preoperatively. Multiple reports have demonstrated inaccuracies in survival assessment by the AJCC system that may, in part, be due to not accounting for depth of tumor invasion (81-84). In fact, retrospective case series have shown that the MSKCC staging more accurately correlates with overall survival than the AJCC system (85,86). Given the various staging systems and the difficulty in comparing HC studies across various centers, DeOliveira and colleagues proposed a new staging strategy that accounts for tumor size, extent of disease in the biliary system, vascular involvement, lymph node involvement, distant metastases, and the volume of the putative remnant liver after resection (87).
Pathological characteristics
Grossly, HC is divided into three classifications; papillary, nodular and sclerosing. Both nodular and papillary subtypes typically protrude into the lumen. Sclerosing HC is diffusely infiltrating along the biliary wall with very little mucosa protuberance on gross examination (88). Sclerosing HC is the most common subtype. Papillary HC is more often resectable and is thought to have the best prognosis due to its less invasive growth pattern (88-90). Many tumors, however, have overlapping features.
Over 90% of extrahepatic epithelial bile duct tumors are adenocarcinomas. These are divided into three grades; well, moderately and poorly differentiated depending on the percentage of glands within the tumor (91,92). Direct invasion into hilar structures as well as lymphatic and perineural invasion is commonly seen. Although typically expressed on the cell membranes of benign biliary epithelium, cytoplasmic CEA and MUC1 expression is often detected in HC and is thought to play a role in the tumor’s metastatic potential (88). Gene expression profiles amongst biliary tract adenocarcinomas differ depending on site of origin and can have prognostic implications. For example, cycle-dependent kinase inhibitor p27 expression was more commonly seen in HC compared with gallbladder and distal bile duct tumors and low expression has correlated with poor outcome (93). K-ras mutations are associated with a worse overall survival in bile duct cancers and are seen in higher frequency in distal bile duct tumors compared with HC (94,95).
Premalignant lesions include biliary intraepithelial neoplasia (BiIN) and intraductal papillary neoplasm of the biliary tract (IPN-B). These lesions are considered counterparts to pancreatic intraepithelial neoplasms (PanIN) and intraductal mucinous neoplasm of the pancreas (IPMN-P), respectively (88,91). Similarly to PanIN, BiIN progress to tubular adenocarcinoma and is graded according to the degree of atypia. BiIN-3 represents carcinoma in situ and is seen in 10-75% of extra hepatic bile duct cancers (88). Superficial spreading along the biliary epithelium is seen in approximately 10-18% of extrahepatic bile duct cancers and is associated with a better prognosis (96,97).
Management
Preoperative liver optimization
A large number of HC patients are jaundiced and hepatic resection in this setting has been associated with increased postoperative complications (54). Therefore, biliary drainage of the FLR should be performed to decrease bilirubin levels thereby facilitating future liver hypertrophy (53). If the FLR is expected to be less than 30-40%, portal vein embolization (PVE) should be considered (53,98,99). Although there are no randomized controlled clinical trials evaluating the effectiveness of PVE in HC, the implementation of PVE seems to allow for the preoperative hypertrophy of the FLR and has been associated with reduced postoperative hepatic failure and complications. Patients are generally eligible for resection 4-6 weeks after biliary drainage and PVE (53,98).
Resection
Criteria for unresectability include bilateral spread to secondary biliary radicals, involvement of the portal vein main trunk, bilobar involvement of hepatic arterial and/or portal venous branches, and unilateral hepatic artery involvement with evidence of extensive contralateral duct spread (100). Hepatic artery and portal vein involvement, peritoneal spread and suspicious lymph nodes on preoperative imaging are important prognostic factors of resectability (37,38,101). Despite the use of various imaging techniques to identify these features, 40-50% of surgically explored HC patients are found to have inoperable disease at the time of laparotomy (13,102). Many have advocated staging laparoscopy in order to prevent unnecessary laparotomies. In some series, staging laparoscopy was able to prevent unnecessary laparotomies in 45% of patients (102-105). The yield and accuracy of the staging laparoscopy may improve with the addition of laparoscopic US (106), however peritoneal washing and cytology provides no benefit (107). Although grade A evidence is lacking, staging laparoscopy in HC is acceptable in selected patients especially those with high CA19-9 levels or indeterminate/suspected extrahepatic disease.
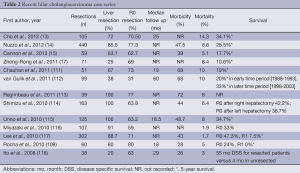
Margin negative (R0) resection remains the only treatment that offers the chance at long-term survival (13-15,17,77,108-110). Surgical resection of HC can sometimes be challenging, however, due to extensive nature and central location of the tumor. Morbidity and mortality rates range from 40% to 70% and 5% to 15%, respectively (Table 2) (13-15,17,113,119). Local excision of only the extrahepatic biliary tree should be avoided, as this approach is associated with a high likelihood of an R1 (microscopic) or R2 (macroscopic) resection, as well as worse lymph node clearance and worse survival (14,110,120-124). Major hepatectomy combined with extra hepatic bile duct resection has increased R0 resection rates as well as long term survival and should be considered standard therapy (17,77,118,120,125). In general, Bismuth-Corlette I, II, IIIa lesions typically require an extended right hepatectomy, while Bismuth-Corlette IIIb lesions require a left hepatectomy. Complete excision of the caudate lobe has also been demonstrated to improve local recurrence rates and long-term survival (100,126-128). As such, routine resection of the caudate lobe should be performed. Lymph node and perineural invasion occur early and are associated with poor survival (88,129). While lymph node dissection does not provide a survival benefit, it may help with local control and is important prognostically as the 5-year survival of patients with positive lymph node disease is 15% (130-132).
Full table
In most surgical series that include hepatic resection combined with excision of the extrahepatic biliary tree, obtaining an R0 (negative microscopic) margin is typically reported in the 60-80% range (13,14,17,113,116,120). Intraoperatively, while frozen section analysis of the margins should be obtained, it has not been shown to improve the probability of margin negative resection (20,21). Although R1 resections have shown some survival benefit compared to non-operative management, an R0 margin should always be the goal (133-135).
The routine use of vascular resection is less defined and more controversial. In one meta-analysis, portal vein resection (PVR) was associated with higher mortality rates, although this association was lost in a subgroup analysis of more experienced centers (136). Importantly, there was no difference in 5-year survival rates between the PVR cohort and the non-vein resection group, despite the fact that the PVR cohort had more advanced disease (136). Overall, PVR has demonstrated long term survival advantage in patients with advanced HC and should not be considered a contraindication to resection (120,137). Conversely, hepatic artery resection is associated with increased morbidity and mortality without an appreciable benefit in long-term survival and is not performed routinely (138,139). In 1999, Neuhaus and colleagues introduced the “no-touch” technique that calls for en bloc right trisectionectomy combined with routine PVR (140). In a recent retrospective review, Neuhaus and colleagues demonstrate a 58% 5-year survival in the no touch technique cohort versus 29% 5-year survival after conventional hepatectomy (P=0.02) (141). Notably, there was no significant difference in operative mortality between the two groups. Although the results are encouraging, these reports suffer from some confounding and selection bias given the retrospective nature of the reports. As such, additional data are necessary before widespread adoption of the “no touch” technique should be adopted as the standard of care for resection of HC.
The role of minimally invasive HC resections also remains unclear. Small-scale case series and singular case reports comprise most of the literature. The available data have demonstrated the feasibility of this technique although larger studies with longer follow up are needed before this technique can be properly assessed (142-144).
Some authors have recommended operative palliation in patients who undergo a laparotomy and are then found to have unresectable disease (145). Surgical palliation in HC consists of cholecystectomy and a biliary-enteric anastomosis for biliary drainage. Although surgical biliary drainage is associated with improved patency rates, there is increased morbidity (17-51%) and mortality (6-12%) along with no difference in overall survival when surgical palliation is compared to non-operative management (146-148). The main complication seen in most series is biliary-enteric anastomotic leak (6-21%) (147). Surgical drainage is therefore not routinely recommended and palliative resections are generally not necessary if the patient has been adequately drained with biliary stenting (147,149).
In general, 5-year survival after surgical resection of HC ranges from 10% to 40% (13,14,150). Of note, even following an R0 resection, recurrence can be as high as 50-70% (151,152). Lymph node metastasis, lymphovascular invasion, positive histologic margins, and higher T stage have all been associated with worse survival and increased recurrence (13-15,111,117,153-155).
Transplantation
Over the last decade, orthotopic liver transplantation (OLT) has shown promise in the treatment of unresectable HC. Multiple early studies evaluating OLT for unresectable HC demonstrated a dismal 20-30% 5-year survival (156-158). The pioneering work from the Mayo Clinic group, however, established a successful multimodality HC OLT protocol and demonstrated a 60% five-year survival in highly selected patients (27,159). Criteria for OLT consideration include HC diagnosis by transluminal biopsy (with percutaneous biopsy being contraindicated), brush cytology, biliary stricture plus FISH polysomy, mass lesion on cross-sectional imaging or malignant—appearing stricture combined with elevated CA19-9 or FISH polysomy (160). Patients must have adequate performance status to withstand neoadjuvant therapy and liver transplantation. Exclusion criteria include patients with a mass lesion below the level of cystic duct, tumors greater than 3 cm, evidence of intrahepatic or extrahepatic metastases, or previous history of transperitoneal biopsy. The treatment protocol consists of external beam radiation (40-45 Gy), transcatheter radiation (20-30 Gy) via ERCP or PTC with radiosensitizing 5-FU followed by oral capecitabine until the day of transplantation (160). Prior to transplantation, patients undergo a staging operation where lymph nodes are excised for evaluation and the abdomen is thoroughly inspected for the presence of metastatic disease. In 2009, the United Network for Organ Sharing (UNOS) recognized HC as an indication for OLT (27,161). As such, other centers have begun to use OLT increasingly for unresectable HC. In a retrospective series including 12 major US transplant centers performing UNOS approved protocol OLT for HC, Darwish Murad et al. demonstrated a 65% recurrence free survival after five years. Moreover, there was no difference in outcomes when evaluating patients transplanted at the Mayo clinic (n=131) versus all other centers (n=83) (27). Finally, when UNOS protocol adhering centers’ outcomes were compared with the results of non-protocol driven centers, protocol adherence remained strongly associated with improved recurrence free survival (27).
The three-year survival for PSC related HC is less than 20%, even after surgical resection (162,163). Moreover, CC arising in the setting of PSC typically presents at an advanced stage with multifocal disease which is not amenable to surgical resection (162). Rea and colleagues reported improved survival with OLT in PSC related HC compared to those who underwent surgical resection (164). In their 12 center multi institution analysis, Darwish Murad and colleagues showed a strong trend toward increased recurrence free survival after ten years in PSC related HC liver transplant compared to OLT in non-PSC related HC (62% vs. 51%, P=0.06) (27). Five-year survival after OLT when combined with neoadjuvant therapy in HC arising in the setting of PSC is over 70% and should therefore be considered the standard of care for this specific patient population (164). In contrast, OLT for non-PSC related HC remains more controversial. Future applications of OLT in HC should encompass stringent protocol driven criteria and further external validation is needed.
Role of radiation therapy
Studies incorporating conventional 5-FU based chemotherapy and radiation for unresectable HC have demonstrated mixed results. The data consists of small single institutional series combining various biliary tree neoplasms (i.e., gall bladder, intrahepatic and extrahepatic bile duct cancer). Prospective HC trials are limited given the tumor’s rarity, aggressiveness and late presentation. In the adjuvant setting, the goal of radiation is to provide local disease control, slow overall disease progression, and prolong survival. Local control of HC following surgery is critical because of the morbidity of local progression in the biliary tract. Chemoradiation may also help prevent or palliate symptoms associated with uncontrolled local progression in both the adjuvant and unresectable setting.
In one retrospective study, Todoroki et al. reported on 63 patients who underwent resection of Klatskin tumors between 1976 and 1999 (165). Overall 29/49 patients were treated in the adjuvant setting with intraoperative radiation therapy (IORT), external beam radiation therapy (EBRT), or a combination of IORT + EBRT. The 5-year survival was 33.9% in the adjuvant radiation arm and 13.5% in the observation arm (P<0.01). Patients who had a combination of EBRT and IORT had better survival. Locoregional failure was decreased in the group that received adjuvant radiation: 20% compared with 69%. Another study by Gerhards et al. reported on 91 patients who underwent mostly margin-positive surgical resection (86%) for HC, of which 71 received EBRT, intraluminal radiation, or a combination. The median survival for those patients who received radiation was 24 months, compared with eight months among those who were simply observed (P<0.01) (166). While retrospective, these studies suggest that adjuvant radiation therapy may improve local control and survival in patients with margin positive resections.
Historically, conventional radiation is delivered over 5-6 weeks and has been limited to approximately 54 Gy because of concern about radiation injury to organs at risk (OARs), especially surrounding bowel and stomach. With a better understanding of dose tolerances of OARs and improved conformality of treatment modalities including intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), radiation has become more widely used (167). Patients with unresectable disease treated with standard fractionated radiotherapy and/or chemotherapy have traditionally had dismal outcomes. SBRT allows for the delivery of higher doses of radiation delivered over a shorter period of time (<2 weeks) and have shown some promise with respect to local recurrence. However, distant failure remains high, underscoring a need for more effective systemic agents.
Role of chemotherapy
The current standard chemotherapy regimens for unresectable HC are platinum based in combination with gemcitabine. These regimens have demonstrated small improvements in survival in randomized control clinical trials as well as in small retrospective series (168-171). Despite the limited data, chemotherapy is indicated for patients with unresectable tumors and adequate functional status (172). Prospective randomized studies are needed and ongoing to fully understand its capabilities, and to optimize the regimen for specific patients, incorporating novel, targeted agents in addition to traditional cytotoxic drugs (173).
Neoadjuvant therapy in HC remains poorly characterized as well. Some studies have demonstrated encouraging outcomes, particularly in the transplant literature (27). Few prospective trials, however, have investigated its ability to downstage tumors and lead to R0 resection (174). More studies are needed and there is no indication to delay tumor resection for neoadjuvant therapy.
Photodymamic therapy
Photodynamic therapy (PDT) involves the intravenous administration of photosensitizing agents which accumulate within cancer cells. Light activation leads to the formation of singlet oxygen free radicals and the destruction of nearby cells. Cutaneous phototoxicity is seen in 30% of patients (175). Multiple prospective and retrospective series have demonstrated an increase in survival of 2-3 months with the addition of PDT to biliary stenting in a palliative setting (176-179). A phase II pilot study by Wiedmann et al. evaluating PDT as a neoadjuvant modality demonstrated a 1-year survival of 83% (180). Although underpowered, it did demonstrate the feasibility of this therapy and calls for future prospective studies (180).
Conclusions
In conclusion, HC is a rare but aggressive disease with a dismal long-term prognosis. Lymph node invasion, tumor grade and negative margins are important prognostic indicators. R0 resection represents the only chance for long-term survival. Local resection should not be undertaken. Standard therapy consists of extrahepatic bile duct resection, hepatectomy and en bloc lymphadenectomy. OLT has demonstrated acceptable outcomes in highly selected patients. Chemotherapy and radiation may improve overall survival although prospective randomized trials are needed. Due to the complexity of this disease, a multi-disciplinary approach with multimodal treatment is recommended.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Altemeier WA, Gall EA, Zinninger MM, et al. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg 1957;75:450-60; discussion 460-1. [PubMed]
- Klatskin G. Adenocarcinoma of the Hepatic Duct at Its Bifurcation within the Porta Hepatis. An Unusual Tumor with Distinctive Clinical and Pathological Features. Am J Med 1965;38:241-56. [PubMed]
- Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. [PubMed]
- Cai WK, Sima H, Chen BD, et al. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2011;17:249-53. [PubMed]
- Ramia JM. Hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:113-4. [PubMed]
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84. [PubMed]
- Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, et al. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:132-8. [PubMed]
- Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873-5. [PubMed]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [PubMed]
- West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer 2006;94:1751-8. [PubMed]
- Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 2004;99:523-6. [PubMed]
- Broomé U, Olsson R, Loof L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610-5. [PubMed]
- Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012;16:1672-9. [PubMed]
- Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012;147:26-34. [PubMed]
- Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB (Oxford) 2012;14:142-9. [PubMed]
- Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 2011;202:310-20. [PubMed]
- Zheng-Rong L, Hai-Bo Y, Xin C, et al. Resection and drainage of hilar cholangiocarcinoma: an 11-year experience of a single center in mainland China. Am Surg 2011;77:627-33. [PubMed]
- Are C, Gonen M, D’Angelica M, et al. Differential diagnosis of proximal biliary obstruction. Surgery 2006;140:756-63. [PubMed]
- Corvera CU, Blumgart LH, Darvishian F, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg 2005;201:862-9. [PubMed]
- Gerhards MF, Vos P, van Gulik TM, et al. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg 2001;88:48-51. [PubMed]
- Koea J, Holden A, Chau K, et al. Differential diagnosis of stenosing lesions at the hepatic hilus. World J Surg 2004;28:466-70. [PubMed]
- Choi BI, Lee JM, Han JK. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging 2004;29:548-57. [PubMed]
- Knoefel WT, Prenzel KL, Peiper M, et al. Klatskin tumors and Klatskin mimicking lesions of the biliary tree. Eur J Surg Oncol 2003;29:658-61. [PubMed]
- Wetter LA, Ring EJ, Pellegrini CA, et al. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg 1991;161:57-62; discussion 62-3. [PubMed]
- Berardi R, Mocchegiani F, Pierantoni C, et al. Resected biliary tract cancers: a novel clinical-pathological score correlates with global outcome. Dig Liver Dis 2013;45:70-4. [PubMed]
- Juntermanns B, Radunz S, Heuer M, et al. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur J Med Res 2010;15:357-61. [PubMed]
- Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88-98.e3; quiz e14.
- Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol 2008;191:1448-57. [PubMed]
- Ruys AT, van Beem BE, Engelbrecht MR, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol 2012;85:1255-62. [PubMed]
- Ruys AT, van Haelst S, Busch OR, et al. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg 2012;36:2179-86. [PubMed]
- Valls C, Ruiz S, Martinez L, et al. Radiological diagnosis and staging of hilar cholangiocarcinoma. World J Gastrointest Oncol 2013;5:115-26. [PubMed]
- Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics 1999;19:1199-218. [PubMed]
- Hann LE, Greatrex KV, Bach AM, et al. Cholangiocarcinoma at the hepatic hilus: sonographic findings. AJR Am J Roentgenol 1997;168:985-9. [PubMed]
- Xu HX, Chen LD, Xie XY, et al. Enhancement pattern of hilar cholangiocarcinoma: contrast-enhanced ultrasound versus contrast-enhanced computed tomography. Eur J Radiol 2010;75:197-202. [PubMed]
- Choi ER, Chung YH, Lee JK, et al. Preoperative evaluation of the longitudinal extent of borderline resectable hilar cholangiocarcinoma by intraductal ultrasonography. J Gastroenterol Hepatol 2011;26:1804-10. [PubMed]
- Watadani T, Akahane M, Yoshikawa T, et al. Preoperative assessment of hilar cholangiocarcinoma using multidetector-row CT: correlation with histopathological findings. Radiat Med 2008;26:402-7. [PubMed]
- Aloia TA, Charnsangavej C, Faria S, et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg 2007;193:702-6. [PubMed]
- Cha JH, Han JK, Kim TK, et al. Preoperative evaluation of Klatskin tumor: accuracy of spiral CT in determining vascular invasion as a sign of unresectability. Abdom Imaging 2000;25:500-7. [PubMed]
- Endo I, Shimada H, Sugita M, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery 2007;142:666-75. [PubMed]
- Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis 2004;24:155-64. [PubMed]
- Chryssou E, Guthrie JA, Ward J, et al. Hilar cholangiocarcinoma: MR correlation with surgical and histological findings. Clin Radiol 2010;65:781-8. [PubMed]
- Masselli G, Manfredi R, Vecchioli A, et al. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol 2008;18:2213-21. [PubMed]
- Hänninen EL, Pech M, Jonas S, et al. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol 2005;46:462-70. [PubMed]
- Park HS, Lee JM, Choi JY, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol 2008;190:396-405. [PubMed]
- Li J, Kuehl H, Grabellus F, et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol 2008;98:438-43. [PubMed]
- Fritscher-Ravens A, Bohuslavizki KH, Broering DC, et al. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun 2001;22:1277-85. [PubMed]
- Reinhardt MJ, Strunk H, Gerhardt T, et al. Detection of Klatskin’s tumor in extrahepatic bile duct strictures using delayed 18F-FDG PET/CT: preliminary results for 22 patient studies. J Nucl Med 2005;46:1158-63. [PubMed]
- Liu F, Li Y, Wei Y, et al. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 2011;56:663-72. [PubMed]
- Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013;100:274-83. [PubMed]
- Grandadam S, Compagnon P, Arnaud A, et al. Role of preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma type III. Ann Surg Oncol 2010;17:3155-61. [PubMed]
- Tsai HM, Chuang CH, Lin XZ, et al. Factors relating to the short term effectiveness of percutaneous biliary drainage for hilar cholangiocarcinoma. World J Gastroenterol 2009;15:5206-10. [PubMed]
- Young AL, Igami T, Senda Y, et al. Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB (Oxford) 2011;13:483-93. [PubMed]
- Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford) 2005;7:252-3. [PubMed]
- Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg 2000;135:302-8. [PubMed]
- Lai EC, Chu KM, Lo CY, et al. Surgery for malignant obstructive jaundice: analysis of mortality. Surgery 1992;112:891-6. [PubMed]
- Clements WD, Diamond T, McCrory DC, et al. Biliary drainage in obstructive jaundice: experimental and clinical aspects. Br J Surg 1993;80:834-42. [PubMed]
- Kennedy TJ, Yopp A, Qin Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009;11:445-51. [PubMed]
- Iacono C, Ruzzenente A, Campagnaro T, et al. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg 2013;257:191-204. [PubMed]
- Tamada K, Ushio J, Sugano K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol 2011;2:203-16. [PubMed]
- Vogl TJ, Schwarz WO, Heller M, et al. Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur Radiol 2006;16:2317-25. [PubMed]
- van der Gaag NA, Kloek JJ, de Castro SM, et al. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg 2009;13:814-20. [PubMed]
- Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc 1995;42:565-72. [PubMed]
- Kubota Y, Takaoka M, Tani K, et al. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. Am J Gastroenterol 1993;88:1700-4. [PubMed]
- Kang MJ, Choi YS, Jang JY, et al. Catheter tract recurrence after percutaneous biliary drainage for hilar cholangiocarcinoma. World J Surg 2013;37:437-42. [PubMed]
- Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010;14:119-25. [PubMed]
- Kim HM, Park JY, Kim KS, et al. Intraductal ultrasonography combined with percutaneous transhepatic cholangioscopy for the preoperative evaluation of longitudinal tumor extent in hilar cholangiocarcinoma. J Gastroenterol Hepatol 2010;25:286-92. [PubMed]
- Shim CS, Cheon YK, Cha SW, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy 2005;37:425-33. [PubMed]
- Parodi A, Fisher D, Giovannini M, et al. Endoscopic management of hilar cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2012;9:105-12. [PubMed]
- Gleeson FC, Rajan E, Levy MJ, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc 2008;67:438-43. [PubMed]
- DeWitt J, Misra VL, Leblanc JK, et al. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc 2006;64:325-33. [PubMed]
- Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol 2004;99:45-51. [PubMed]
- Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356-60. [PubMed]
- Lee SS, Kim MH, Lee SK, et al. MR cholangiography versus cholangioscopy for evaluation of longitudinal extension of hilar cholangiocarcinoma. Gastrointest Endosc 2002;56:25-32. [PubMed]
- Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31-8. [PubMed]
- Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170-8. [PubMed]
- Paul A, Kaiser GM, Molmenti EP, et al. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg 2011;77:1695-9. [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [PubMed]
- Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005;241:693-9; discussion 699-702. [PubMed]
- Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998;228:385-94. [PubMed]
- Cancer Staging Manual. 7th ed. American Joint Committee on Cancer. NY, USA: Springer, 2010.
- de Jong MC, Hong SM, Augustine MM, et al. Hilar cholangiocarcinoma: tumor depth as a predictor of outcome. Arch Surg 2011;146:697-703. [PubMed]
- Zervos EE, Osborne D, Goldin SB, et al. Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg 2005;190:810-5. [PubMed]
- Hidaka A. Clinicopathological study of patients undergoing resection of hilar cholangiocarcinoma. Kurume Med J 2007;54:41-9. [PubMed]
- Nagahashi M, Shirai Y, Wakai T, et al. Depth of invasion determines the postresectional prognosis for patients with T1 extrahepatic cholangiocarcinoma. Cancer 2010;116:400-5. [PubMed]
- Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343-55. [PubMed]
- Zaydfudim VM, Clark CJ, Kendrick ML, et al. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am J Surg 2013;206:159-65. [PubMed]
- Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363-71. [PubMed]
- Castellano-Megías VM, Ibarrola-de Andres C, Colina-Ruizdelgado F. Pathological aspects of so called “hilar cholangiocarcinoma”. World J Gastrointest Oncol 2013;5:159-70. [PubMed]
- Jarnagin WR, Bowne W, Klimstra DS, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg 2005;241:703-12; discussion 712-4. [PubMed]
- Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656-65. [PubMed]
- Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO Classification of Tumors of the Digestive System. 4th ed. Lyon: IARC, 2010.
- Washington MK, Berlin J, Branton PA, et al. Protocol for the examination of specimens from patients with carcinoma of the distal extrahepatic bile ducts. Arch Pathol Lab Med 2010;134:e8-13. [PubMed]
- Jarnagin WR, Klimstra DS, Hezel M, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol 2006;24:1152-60. [PubMed]
- Rashid A, Ueki T, Gao YT, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res 2002;8:3156-63. [PubMed]
- Ohashi K, Tstsumi M, Nakajima Y, et al. Ki-ras point mutations and proliferation activity in biliary tract carcinomas. Br J Cancer 1996;74:930-5. [PubMed]
- Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 1998;227:405-11. [PubMed]
- Nakanishi Y, Zen Y, Kawakami H, et al. Extrahepatic bile duct carcinoma with extensive intraepithelial spread: a clinicopathological study of 21 cases. Mod Pathol 2008;21:807-16. [PubMed]
- Yokoyama Y, Nagino M, Nishio H, et al. Recent advances in the treatment of hilar cholangiocarcinoma: portal vein embolization. J Hepatobiliary Pancreat Surg 2007;14:447-54. [PubMed]
- Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208-17. [PubMed]
- Parikh AA, Abdalla EK, Vauthey JN. Operative considerations in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:254-8. [PubMed]
- Ruys AT, Busch OR, Rauws EA, et al. Prognostic impact of preoperative imaging parameters on resectability of hilar cholangiocarcinoma. HPB Surg 2013;2013:657309.
- Ruys AT, Busch OR, Gouma DJ, et al. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol 2011;18:2647-53. [PubMed]
- Connor S, Barron E, Wigmore SJ, et al. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg 2005;9:476-80. [PubMed]
- Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002;235:392-9. [PubMed]
- Barlow AD, Garcea G, Berry DP, et al. Staging laparoscopy for hilar cholangiocarcinoma in 100 patients. Langenbecks Arch Surg 2013;398:983-8. [PubMed]
- Joseph S, Connor S, Garden OJ. Staging laparoscopy for cholangiocarcinoma. HPB (Oxford) 2008;10:116-9. [PubMed]
- Martin RC 2nd, Fong Y, DeMatteo RP, et al. Peritoneal washings are not predictive of occult peritoneal disease in patients with hilar cholangiocarcinoma. J Am Coll Surg 2001;193:620-5. [PubMed]
- Schiffman SC, Reuter NP, McMasters KM, et al. Overall survival peri-hilar cholangiocarcinoma: R1 resection with curative intent compared to primary endoscopic therapy. J Surg Oncol 2012;105:91-6. [PubMed]
- Rocha FG, Matsuo K, Blumgart LH, et al. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci 2010;17:490-6. [PubMed]
- Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery 1998;123:131-6. [PubMed]
- Chauhan A, House MG, Pitt HA, et al. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB (Oxford) 2011;13:139-47. [PubMed]
- van Gulik TM, Ruys AT, Busch OR, et al. Extent of liver resection for hilar cholangiocarcinoma (Klatskin tumor): how much is enough? Dig Surg 2011;28:141-7. [PubMed]
- Regimbeau JM, Fuks D, Le Treut YP, et al. Surgery for hilar cholangiocarcinoma: a multi-institutional update on practice and outcome by the AFC-HC study group. J Gastrointest Surg 2011;15:480-8. [PubMed]
- Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg 2010;251:281-6. [PubMed]
- Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2010;17:463-9. [PubMed]
- Miyazaki M, Kimura F, Shimizu H, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J Hepatobiliary Pancreat Sci 2010;17:470-5. [PubMed]
- Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010;17:476-89. [PubMed]
- Ito F, Agni R, Rettammel RJ, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg 2008;248:273-9. [PubMed]
- van Gulik TM, Kloek JJ, Ruys AT, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 2011;37:65-71. [PubMed]
- de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer 2012;118:4737-47. [PubMed]
- Ikeyama T, Nagino M, Oda K, et al. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg 2007;246:1052-7. [PubMed]
- Neuhaus P, Jonas S, Settmacher U, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg 2003;388:194-200. [PubMed]
- Capussotti L, Vigano L, Ferrero A, et al. Local surgical resection of hilar cholangiocarcinoma: is there still a place? HPB (Oxford) 2008;10:174-8. [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [PubMed]
- Shi Z, Yang MZ, He QL, et al. Addition of hepatectomy decreases liver recurrence and leads to long survival in hilar cholangiocarcinoma. World J Gastroenterol 2009;15:1892-6. [PubMed]
- Cheng QB, Yi B, Wang JH, et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 2012;38:1197-203. [PubMed]
- Kow AW, Wook CD, Song SC, et al. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 2012;36:1112-21. [PubMed]
- Dinant S, Gerhards MF, Busch OR, et al. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7:263-7. [PubMed]
- Ramacciato G, Nigri G, Bellagamba R, et al. Univariate and multivariate analysis of prognostic factors in the surgical treatment of hilar cholangiocarcinoma. Am Surg 2010;76:1260-8. [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Prognostic significance of lymph node ratio after resection of peri-hilar cholangiocarcinoma. HPB (Oxford) 2011;13:240-5. [PubMed]
- Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 2010;251:675-81. [PubMed]
- Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg 2001;233:385-92. [PubMed]
- Lee JH, Hwang DW, Lee SY, et al. The proximal margin of resected hilar cholangiocarcinoma: the effect of microscopic positive margin on long-term survival. Am Surg 2012;78:471-7. [PubMed]
- Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol 2008;34:787-94. [PubMed]
- Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg 2007;204:250-60. [PubMed]
- Wu XS, Dong P, Gu J, et al. Combined portal vein resection for hilar cholangiocarcinoma: a meta-analysis of comparative studies. J Gastrointest Surg 2013;17:1107-15. [PubMed]
- Hemming AW, Mekeel K, Khanna A, et al. Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg 2011;212:604-13; discussion 613-6. [PubMed]
- Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery 2007;141:581-8. [PubMed]
- Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford) 2013;15:492-503. [PubMed]
- Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999;230:808-18; discussion 819. [PubMed]
- Neuhaus P, Thelen A, Jonas S, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol 2012;19:1602-8. [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic extended right hepatectomy with biliary reconstruction. J Laparoendosc Adv Surg Tech A 2010;20:159-63. [PubMed]
- Gumbs AA, Jarufe N, Gayet B. Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg Endosc 2013;27:406-14. [PubMed]
- Yu H, Wu SD, Tian Y, et al. Single-incision laparoscopic resection of Bismuth I hilar cholangiocarcinoma. Surg Innov 2013;20:209-13. [PubMed]
- Gerhards MF, den Hartog D, Rauws EA, et al. Palliative treatment in patients with unresectable hilar cholangiocarcinoma: results of endoscopic drainage in patients with type III and IV hilar cholangiocarcinoma. Eur J Surg 2001;167:274-80. [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Unresectable hilar cholangiocarcinoma: multimodality approach with percutaneous treatment associated with radiotherapy and chemotherapy. In Vivo 2006;20:757-60. [PubMed]
- Singhal D, van Gulik TM, Gouma DJ. Palliative management of hilar cholangiocarcinoma. Surg Oncol 2005;14:59-74. [PubMed]
- Li HM, Dou KF, Sun K, et al. Palliative surgery for hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2003;2:110-3. [PubMed]
- Connor S, Barron E, Redhead DN, et al. Palliation for suspected unresectable hilar cholangiocarcinoma. Eur J Surg Oncol 2007;33:341-5. [PubMed]
- Shimizu H, Sawada S, Kimura F, et al. Clinical significance of biliary vascular anatomy of the right liver for hilar cholangiocarcinoma applied to left hemihepatectomy. Ann Surg 2009;249:435-9. [PubMed]
- Kobayashi A, Miwa S, Nakata T, et al. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56-64. [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [PubMed]
- Patel SH, Kooby DA, Staley CA 3rd, et al. The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: a new selection criterion for adjuvant therapy? HPB (Oxford) 2011;13:605-11. [PubMed]
- Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg 1999;230:663-71. [PubMed]
- Klempnauer J, Ridder GJ, Werner M, et al. What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer 1997;79:26-34. [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [PubMed]
- Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 2004;239:265-71. [PubMed]
- Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl 2005;11:1412-6. [PubMed]
- Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis 2004;24:201-7. [PubMed]
- Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am 2009;18:325-37. [PubMed]
- Darwish Murad S, Heimbach JK, Gores GJ, et al. Excellent quality of life after liver transplantation for patients with perihilar cholangiocarcinoma who have undergone neoadjuvant chemoradiation. Liver Transpl 2013;19:521-8. [PubMed]
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660-78. [PubMed]
- Rosen CB, Nagorney DM, Wiesner RH, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg 1991;213:21-5. [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8; discussion 458-61. [PubMed]
- Todoroki T, Ohara K, Kawamoto T, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys 2000;46:581-7. [PubMed]
- Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg 2003;27:173-9. [PubMed]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651-8. [PubMed]
- Murakami Y, Uemura K, Sudo T, et al. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. J Gastrointest Surg 2009;13:1470-9. [PubMed]
- Dumitrascu T, Chirita D, Ionescu M, et al. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 2013;17:913-24. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- Yang R, Wang B, Chen YJ, et al. Efficacy of gemcitabine plus platinum agents for biliary tract cancers: a meta-analysis. Anticancer Drugs 2013;24:871-7. [PubMed]
- Faris JE, Zhu AX. Targeted therapy for biliary tract cancers. J Hepatobiliary Pancreat Sci 2012;19:326-36. [PubMed]
- Grendar J, Grendarova P, Sinha R, et al. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: a systematic review. HPB (Oxford) 2013. [Epub ahead of print].
- Petersen BT, Chuttani R, Croffie J, et al. Photodynamic therapy for gastrointestinal disease. Gastrointest Endosc 2006;63:927-32. [PubMed]
- Cheon YK, Lee TY, Lee SM, et al. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB (Oxford) 2012;14:185-93. [PubMed]
- Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230-9. [PubMed]
- Dumoulin FL, Gerhardt T, Fuchs S, et al. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest Endosc 2003;57:860-7. [PubMed]
- Quyn AJ, Ziyaie D, Polignano FM, et al. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11:570-7. [PubMed]
- Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer 2003;97:2783-90. [PubMed]


