
Fasciola hepatica in a country of low incidence: a tricky diagnosis
Introduction
Fascioliasis is a trematode zoonotic infection caused by two parasites, Fasciola hepatica and Fasciola gigantica (1). Whereas Fasciola hepatica is present worldwide, Fasciola gigantica seems restricted to Africa and Asia (2). Worldwide an estimated 2.5 to 17 million people are infected, with 89 million people at risk (2). Human infection with Fasciola is influenced by environmental characteristics, meaning the incidence in some other countries is much higher than in Switzerland. A province in Peru has even been selected as representative of a human hyperendemic region, where up to 47.7% of school children are infected (1-3).
The life cycle is complex, involving a final host where the adult parasite matures and eggs are produced. It also includes an intermediate host where the larval form develops (4). Final hosts are a wide range of mammals, including ruminants such as cattle and sheep, as well as human beings (5). Through ingestion of contaminated fresh water, vegetables or from grazing on infected pastures, the newly excysted juvenile migrates through the intestinal wall to the peritoneal cavity and into the liver parenchyma, where it matures and enters the bile ducts as an adult fluke (4). Symptoms may be unspecific. The incubation period can last 3–4 months and is asymptomatic. In the invasive or acute phase, fever, abdominal pain, unspecific gastrointestinal symptoms, urticaria and pruritus or respiratory symptoms and eosinophilia may appear. The subsequent latent phase, where the parasite migrates within the liver parenchyma, is either asymptomatic at a low infection dose, or—at a high infection dose—accompanied by eosinophilia and/or gastrointestinal complaints. Due to destruction of liver parenchyma, liver enzymes (Aspartat-Aminotransferase or Alanin-Aminotransferase) may be elevated. In the biliary or chronic phase, the adult fluke causes thickening and dilatation of the bile ducts, resulting in upper abdominal pain, weight loss and fatigue (6,7).
Diagnosis is made by stool sample analysis (direct parasitological technique), immunological tests and/or imaging, combined with the need for a high clinical suspicion. On imaging, multiple subcapsular nodules with branching lesions in the liver, dilatation of the bile ducts or wall thickening of the bile ducts or gallbladder may be observed (8-10).
In Switzerland, Fasciola hepatica is still rare in humans. The first case was reported in 1936 in a traveller returning from Sumatra (11), followed by a few isolated case-reports of mostly imported cases diagnosed after a trip abroad (12-15). In 2009, a rise in the incidence of positive serologies for Fasciola hepatica with 22 cases diagnosed within a 2-month period prompted a federal inquest into a suspected epidemic in central Switzerland. Although no definitive cause could be determined, a correlation with ingestion of watercress was suspected (16).
Patients are likely to undergo multiple, unnecessary investigations and treatments, sometimes for years, before a correct diagnosis is made, as most physicians in our country have never met this disease during their career due to its low incidence. With some patients presenting with jaundice and weight loss and imaging showing abnormal bile ducts or the presence of a liver lesion, Fasciola hepatica may be misdiagnosed as cholangiocarcinoma or another malignant liver lesion. This in turn may result in the patient undergoing unnecessary, complex liver surgery.
Here we present our experience with Fasciola hepatica in a retrospective analysis of all cases diagnosed at our institution.
Methods
We conducted a retrospective analysis of all patients with Fasciola hepatica diagnosed at the University Hospital of Bern, Inselspital, Switzerland between 2005 and 2018. Our aim was to determine how frequently this disease was diagnosed in a tertiary, university hospital in Switzerland and to evaluate the outcome after treatment. The decision to test for Fasciola hepatica was determined by the treating physician. Personal data, laboratory results, imaging, treatment modality and outcome were collected from patient files and anonymized.
Sixty patients had a positive serology for Fasciola hepatica between 2005 and 2018. Forty-four patients were excluded as they did not meet the diagnostic criteria described below. Two further patients were excluded for lack of medical records and one patient did not give consent to be included in the analysis. Final evaluation was carried out in thirteen patients.
The study protocol was approved by the Regional Ethical Review Boards in Bern (KEK-Nr. 2018-02056)
Diagnostic criteria
Diagnosis of Fasciola hepatica infection was made when a positive serology and/or stool analysis for parasite eggs correlated with clinical suspicion, based on symptoms and imaging of the liver and bile ducts. All patients with suspected infection subsequently received treatment. Clinical suspicion of Fasciola hepatica was defined as eosinophilia or elevated liver enzymes or cholestasis, with imaging such as ultrasound or computed tomography (CT) showing dilated or thickened bile ducts or liver lesions.
All patient files with positive serologies were reviewed by an infectious disease specialist. Patients were excluded and serology was considered false positive if no treatment for Fasciola hepatica was given for the following reasons: (I) only weakly positive Fasciola hepatica serology; (II) a serology positive for more than one parasite where an infection with another parasite was deemed more likely (low levels of Fasciola hepatica serum antibodies, higher levels of antibodies for other helminthic parasites) or (III) unspecific symptoms and the presence of another, more likely (non-parasitic) diagnosis.
Serology
Diagnosis of Fasciola hepatica was determined by serology using Excretory-Secretory products (FhE/S) from Fasciola hepatica. An FhE/S-Enzyme linked immunosorbent assay (ELISA) detected the total circulating serum immunoglobulins G (IgG). The sensitivity of FhE/S-ELISA is 93%, with a specificity of 95%. The positive control was from a patient suffering from fascioliasis and exhibiting a positive coprological identification of Fasciola hepatica eggs upon conventional flotation technique. Our methods for the ELISA, the preparation of the antigen, and its sensitivity and specificity have been previously described (17,18).
Parasitological technique
Three stool samples were obtained per patient on three different days. Identification of Fasciola hepatica eggs was done on sodium acetate acetic acid formalin (SAF) stool samples. For increased yield of ova, the stool was concentrated and a wet mount was prepared for microscopy.
Statistical analysis
Descriptive statistics were used, including mean and standard deviation and median and range. The analyses carried out using a commercially available software (SPSS version 25, IBM, Chicago, USA).
Results
Patient characteristics are described in Table 1, symptoms and laboratory values are presented in Table 2. The majority of patients had no significant comorbidities (10/13). Three patients had an atopic predisposition and one patient an intraductal papillary neoplasia of the bile duct diagnosed at the same time as the infection with Fasciola hepatica. One patient had an eosinophilic syndrome of unknown denomination with an eosinophilia, which did not resolve after treatment of the Fasciola hepatica infection. Three patients simultaneously tested positive for other parasites. In these three patients, diagnosis of Fasciola hepatica was considered more likely, as their symptoms did not match the other infection. All three patients were treated for fascioliasis and did not receive any additional treatment for the other parasite. Four patients were asymptomatic (4/13, 30.8%), the others had unspecific symptoms such as fatigue, weight loss and right upper quadrant abdominal pain. Three patients were diagnosed following allergological investigations for generalized pruritus. The duration of symptoms was long before definitive diagnosis was obtained. Average time to diagnosis was 8 months, with one patient being seen for 4 years until correct diagnosis was achieved.
Full table
Full table
The origin of the infection remained uncertain for nine of the patients. In four patients the infection was linked to ingestion of watercress or vegetables grown in close contact to sheep. We suspect only three patients of having acquired the disease during trips abroad in endemic areas (Asia, Italy, South-America).
Only 38% of patients had a documented eosinophilia. All patients gave three stool samples for parasitic egg detection, none of which were positive. All patients had positive serologies but with a wide array of levels, ranging from weakly positive to strongly positive. Seven patients presented with cholestasis, and only four patients had elevated liver enzymes. Six patients (46.2%) had normal liver enzymes and cholestasis parameters.
The findings on imaging are presented in Table 3. Ultrasound was the most frequent initial diagnostic tool, used in 7/13 patients (53.8%), followed by a CT or a magnetic resonance imaging (MRI). The most common finding was a dilatation of the intra- or extra-hepatic bile ducts. Three patients presented with hepatic lesions of unknown origin. Two of these patients underwent surgical resection for suspected metastases, and one had a diagnostic laparoscopy with lymph node biopsies for a suspected Klatskin tumour. In these three cases, histology showed necrosis with epithelioid and giant-cell reactions and eosinophilia, leading to the suspicion of a parasitic infection and the diagnosis of Fasciola hepatica. Parasites were never found in the resected specimen or during endoscopic retrograde cholangiopancreatography (ERCP).
Full table
Treatment consisted of Triclabendazole 10 mg/kg. Eleven patients received two doses on consecutive days, and three patients one dose.
Follow-up was variable. One patient underwent MRI examination every 6 months for 18 months, three had follow-up ultrasounds after 6 months and seven patients had no follow-up imaging. One patient initially needed regular ERCPs with stent placement for a main bile duct stenosis caused by the Fasciola hepatica infection. Lastly, one patient known for HIV and Hepatitis C died of generalized sepsis with Staphylococcus aureus 1 month after treatment. In this case, the patient was initially hospitalized for severe Staphylococcus aureus sepsis, Fasciola hepatica was diagnosed during the work-up.
One patient initially treated with one dose of Triclabendazole needed a second course of Triclabendazole after 2 months, as some abdominal pain persisted and the follow-up serology was persistently high. Disease recurrence was not observed in our patient population.
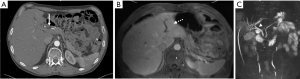
One particular case illustrates the difficulty in diagnosing Fasciola hepatica in a country of low incidence, and is worthy of a detailed report. A 61-year-old male presented with unspecific, mainly postprandial periumbilical pain, unexplained weight loss of 16 kilograms in 1 year and fatigue, accompanied by elevated alkaline phosphatase and transaminases, eosinophilia and an elevated Calprotectin. Over the course of 3 years, multiple investigations were performed. Stool cultures and parasitology (Giardia lamblia, helminths and other protozoa) were negative. A gastroscopy revealed a gastritis and eosophageal reflux grade 1A, colonoscopy was without findings. The patient also suffered from multiple progressive vascular stenoses, including stenosis of the first jejunal branch of the superior mesenteric artery, diagnosed on CT (Figure 1A). Chronic mesenteric ischaemia was suspected. A magnetic resonance cholangiopancreatography (MRCP) was conducted for persistent abdominal complaints, elevated liver enzymes and cholestasis. MRCP revealed a common bile duct stenosis at the hilum with dilatation of the left biliary ducts and atrophy of the left liver (Figure 1B,C). Endoscopic ultrasound and ERCP were very suggestive of a Klatskin tumour, possibly in the setting of primary sclerosing cholangitis. A papillotomy and stenting of the common bile duct were performed, as well as brushing. Although cytology could not confirm malignancy, a hilar cholangiocarcinoma was suspected, also in view of increasing B-symptoms. At this time point, the patient was referred to our clinic. He underwent diagnostic laparoscopy, which excluded a peritoneal carcinomatosis, and an exploratory laparotomy. Cholecystectomy was performed revealing an abnormally thickened cystic duct. Multiple biopsies of the liver and pancreas as well as resection of enlarged hilar and mesenteric lymph nodes were carried out. Pathology did not confirm a tumour, but showed necrosis and eosinophilia, leading to the suspicion of a parasitic infection. Serology was highly positive for the Fasciola Hepatica antigen and the patient was successfully treated with two doses of Triclabendazole.
Discussion
In the past 14 years, 13 cases of Fasciola hepatica were diagnosed in our University Hospital. Most patients presented with unspecific symptoms or were asymptomatic and the delay in diagnosis was significant as long as 4 years in one case. Only four patient had symptoms for less than 3 months prior to diagnosis. Because Fasciola hepatica is rare in humans in Switzerland, patients often go undiagnosed for too long.
Of the 60 positive serologies, 73.3% (n=44) were considered false positive. This is high, and is explained by a combination of cross-reactions with other parasitosis, low antibody levels, and the general low incidence of Fasciola hepatica. An infection with other helminthic parasites is far more likely in Switzerland. In case of an infection with another parasite, this was treated first, before considering treatment of Fasciola hepatica. With all these patients, symptoms resolved once the other parasite had been adequately treated, leaving no need for the treatment of the positive Fasciola serology.
Although typical findings on imaging have been described (8-10), Fasciola hepatica was never suspected on imaging alone. In one case, and after serological diagnosis of Fasciola, a retrospective analysis of the CT showed typical signs, which had been missed on initial presentation.
Most patients were treated with a two-day regimen of Triclabendazole. Of the three patients treated with one dose only, one patient needed a second dose after 2 months. We have no indication of Fasciola hepatica resistant to Triclabendazole in Swiss patients, as can be seen in other countries where the incidence of infection is higher (19).
General awareness of this disease entity is low in Switzerland, resulting in many, mostly unnecessary, investigations. Suspicion for Fasciola hepatica infections needs to be high particularly in the setting of young patients where young age, symptoms and radiological imaging are inconsistent with liver metastases or cholangiocarcinoma.
In our institution, an expert centre for the treatment of alveolar echinococcosis, we are fortunate to have the expertise from the Institute for Parasitology of the Veterinary Faculty at the University of Bern. Working closely with the Institute for Parasitology has increased our awareness of the more uncommon findings associated with parasites of the liver and bile ducts. We therefore tend to test for Fasciola hepatica more often. But even so, we suspect that the actual incidence of Fasciola hepatica infection might be much higher than currently observed.
Up until 2009, the reported cases were all imported, whether by immigration or travel abroad. The epidemic demonstrated the growing incidence of Fasciola hepatica, and the importance of locally acquired infections (16). In our series, we suspect that only three patients acquired the infection abroad. The other infections were linked to watercress consumption or were of unknown/undetermined origin. The prevalence of Fasciola hepatica in cattle in Switzerland is 8.4–21.4% (20,21), and interactive maps have been developed to track the potential risk for liver flukes in different regions (22,23). Our colleagues of the veterinary institute are far more aware of this entity as we are, making our collaboration all the more important.
This study is limited by its retrospective design. Furthermore, many patients diagnosed in our institution were lost to follow up in the long-term as they were treated in other hospitals.
Conclusions
Although infections with Fasciola hepatica are still rare in Switzerland, we expect a rise in its incidence in the following years. This is possibly also due to increased testing following a growing awareness of this illness within the medical professionals, be that due to infections acquired abroad while travelling or locally acquired infections.
Making sure that general practitioners as well as hospital staff (including emergency department physicians) are aware of this illness is essential in a country with a low incidence, if patients are to be treated more efficiently and with significantly reduced delay.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Regional Ethical Review Boards in Bern (KEK-Nr. 2018-02056).
References
- Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol 2005;79:207-16. [Crossref] [PubMed]
- Mas-Coma S, Valero MA, Bargues MD. Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol 2009;69:41-146. [Crossref] [PubMed]
- Gonzalez LC, Esteban JG, Bargues MD, et al. Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop 2011;120:119-29. [Crossref] [PubMed]
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol 2005;35:1255-78. [Crossref] [PubMed]
- Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018;145:1665-99. [Crossref] [PubMed]
- Ashrafi K, Bargues MD, O'Neill S, et al. Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Travel Med Infect Dis 2014;12:636-49. [Crossref] [PubMed]
- Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp Med Biol 2014;766:77-114. [Crossref] [PubMed]
- Behar JM, Winston JS, Borgstein R. Hepatic fascioliasis at a London hospital--the importance of recognising typical radiological features to avoid a delay in diagnosis. Br J Radiol 2009;82:e189-93. [Crossref] [PubMed]
- Sezgin O, Altintas E, Disibeyaz S, et al. Hepatobiliary fascioliasis: clinical and radiologic features and endoscopic management. J Clin Gastroenterol 2004;38:285-91. [Crossref] [PubMed]
- Carnevale S, Malandrini JB, Pantano ML, et al. Fasciola hepatica infection in humans: overcoming problems for the diagnosis. Acta Parasitol 2016;61:776-83. [Crossref] [PubMed]
- Bürgi K. Ein Fall von Leberdistomatose (Fasciola hepatica). Kasuistik und Klinik dieser Erkrankung. Mitt Grenzg Med Chir 1936;44:488-537.
- Helfenstein E, Kayser S, Locher G. Human fascioliasis symptoms. A case report with review of the literature. Praxis (Bern 1994) 2000;89:1427-35.
- Nuesch R, Gremmelmaier D, Hirsch HH, et al. Chest pain after air travel. Lancet 2005;365:1902. [Crossref] [PubMed]
- Andresen B, Blum J, von Weymarn A, et al. Hepatic fascioliasis: report of two cases. Eur Radiol 2000;10:1713-5. [Crossref] [PubMed]
- Markwalder K, Koller M, Goebel N, et al. Schweiz Med Wochenschr 1988;118:1048-52. [Fasciola hepatica infection. Successful therapy using triclabendazole]. [PubMed]
- Health FOoP. Erkrankungen bei Menschen durch Befall mit dem grossen Leberegel (Fasciola hepatica). FOPH Bulletin, 2009:904-10.
- Gottstein B, Jacquier P, Bresson-Hadni S, et al. Improved primary immunodiagnosis of alveolar echinococcosis in humans by an enzyme-linked immunosorbent assay using the Em2plus antigen. J Clin Microbiol 1993;31:373-6. [PubMed]
- Gottstein B, Schneeberger M, Boubaker G, et al. Comparative assessment of ELISAs using recombinant saposin-like protein 2 and recombinant cathepsin L-1 from Fasciola hepatica for the serodiagnosis of human Fasciolosis. PLoS Negl Trop Dis 2014;8:e2860. [Crossref] [PubMed]
- Cabada MM, Lopez M, Cruz M, et al. Treatment Failure after Multiple Courses of Triclabendazole among Patients with Fascioliasis in Cusco, Peru: A Case Series. PLoS Negl Trop Dis 2016;10:e0004361. [Crossref] [PubMed]
- Ducommun D, Pfister K. Prevalence and distribution of Dicrocoelium dendriticum and Fasciola hepatica infections in cattle in Switzerland. Parasitol Res 1991;77:364-6. [Crossref] [PubMed]
- Eckert J, Sauerlaender R, Wolff K. Incidence and geographic distribution of Fasciola hepatica in Switzerland. Schweizer Archiv für Tierheilkunde 1975;117:173-84. [PubMed]
- Knubben-Schweizer G, Deplazes P, Torgerson PR, et al. Bovine fasciolosis in Switzerland: relevance and control. Schweiz Arch Tierheilkd 2010;152:223-9. [Crossref] [PubMed]
- Rapsch C, Dahinden T, Heinzmann D, et al. An interactive map to assess the potential spread of Lymnaea truncatula and the free-living stages of Fasciola hepatica in Switzerland. Vet Parasitol 2008;154:242-9. [Crossref] [PubMed]


