
Neoplastic polyps in gallbladder: a retrospective study to determine risk factors and treatment strategy for gallbladder polyps
Introduction
Gallbladder polyps (GBPs) are lesions that project from the gallbladder wall into the lumen. The widespread use of abdominal imaging techniques has led to a dramatic increase in the diagnosis of GBP (1-4). GBPs are broadly classified as non-neoplastic and neoplastic polyps (5,6). Cholesterol polyps are the most common type of non-neoplastic polyps. Some of the benign neoplastic polyps such as adenomas are considered pre-malignant lesions. Adenomas constitute 10% of GBPs and are found in approximately 1% of cholecystectomy specimens (7).
Current guidelines for management of GBPs are mainly focused on their size; diameter ≥1 cm is the most commonly accepted indication for cholecystectomy (1,8-11). However, a considerable proportion of resected GBPs that are ≥1 cm in size turn out to be non-neoplastic polyps. Moreover, there are few reported cases of malignant transformation of GBPs sized <1 cm (12-16). Hence, some clinicians hesitate to recommend surgery based on the guideline of diameter ≥1 cm.
Malignant polyps account for 1–20% of the resected GBPs (11,17-19). Owing to the poor prognosis of gallbladder carcinoma, early diagnosis of malignant polyps and GBPs with malignant potential is a key imperative. Several studies have sought to identify risk factors that can help differentiate benign from malignant polyps preoperatively (10,11,20-22). Previous reports defined all gallbladder carcinomas as malignant polyps, which may not reflect the real possibility of malignant transformation of GBPs. Differentiation between malignant and benign lesions, as well as between neoplastic and non-neoplastic polyps is a clinical challenge.
We retrospectively evaluated clinical and sonographic data of patients with pathologically-proven GBPs after cholecystectomy over a 14-year period at the China-Japan Friendship Hospital. The objective was to identify the risk factors for neoplastic polyps and provide a precise management strategy for GBPs to avoid unnecessary cholecystectomy without the risk of missing premalignant and malignant polyps.
Methods
Patients and design
We collected and analyzed data of patients with pathologically proven GBP or gallbladder carcinoma after cholecystectomy between January 2003 and December 2016 at the Department of General Surgery, China-Japan Friendship Hospital. Patients with incomplete clinical or sonographic data and those with gallbladder carcinoma without residual adenomatous tissue in pathologic specimen were excluded.
A total of 686 patients with GBPs or adenoma canceration were enrolled in this study. According to histopathology results, patients were classified into three groups: non-neoplastic polyp group (n=542); benign neoplastic polyp group (n=134; referred to as adenoma); adenoma canceration group (n=10). The detailed algorithm for enrollment and grouping of patients is shown in Figure 1. The study protocol was approved by the ethics committee of the China-Japan Friendship Hospital.
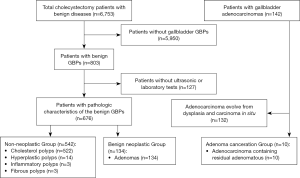
Medical records of patients including clinical features, ultrasound findings, and results of laboratory investigations and histopathological examination were retrospectively reviewed. Clinical features included age, sex, duration between diagnosis and surgery, symptoms, and presence of diabetes mellitus (DM). Ultrasound reports were retrospectively reviewed by an expert and data pertaining to size and number of polyps, echo pattern, and blood flow were recorded. Laboratory data included the routine chemistry panel, fasting blood glucose level, lipid profile, hepatitis virus panel, and tumor markers. Pathologic findings were assimilated from the original reports.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation (SD) or as median (25th percentile, 75th percentile). Multigroup comparison of enumeration data were performed using one-way Analysis of Variance (ANOVA). Non-normally distributed variables were first ranked. For two group comparison, normally distributed variables were compared using Student’s t-test for two independent samples. The Mann-Whitney U test was used for non-normally distributed variables. Categorical variables were summarized as frequencies and percentages, and intergroup comparisons were conducted using Chi-squared or Fisher’s exact test. Variables that showed a significant association on univariate analysis were included in the multivariate binary logistic regression analysis to identify significant predictors of neoplastic polyps; P<0.05 were considered statistically significant.
Results
Demographic and clinical characteristics
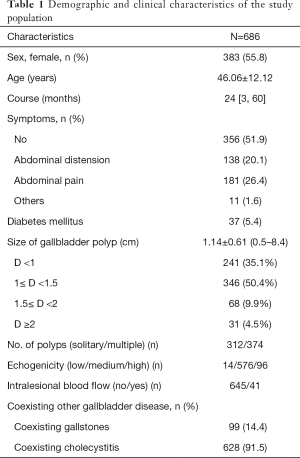
The demographic and clinical characteristics of 686 patients [303 (44.2%) males and 383 (55.8%) females] are summarized in Table 1. The mean age of patients was 46.06±12.12 years; 77.3% patients were in the age-group of 31–60 years. The median time between diagnosis and hospital admission for surgery was 24 months. Approximately half of all patients showed no symptoms at the time of presentation; GBPs were detected on routine check-ups.
Full table
Based on sonography findings, the mean diameter of polyps was 1.14±0.61 cm (range, 0.5–8.4). Three hundred twelve (45.5%) patients had solitary polyps, while 374 (54.5%) patients had multiple polyps. Intralesional blood flow was observed in 41 (2.9%) patients. Out of the 686 patients, 37 (5.4%) had DM.
Histopathologic characteristics of GBPs
Cholesterol polyps were the most common type of GBPs [522 patients (76.1%)]; non-neoplastic polyps also included 20 patients with hyperplastic polyp, inflammatory polyp, or fibrous polyp. Adenomas were found in 134 patients (19.5%) while 10 patients had adenoma canceration. The overall prevalence of malignant polyps was 1.5% (10/686); however, the prevalence increased to 7.0% (10/144) after exclusion of non-neoplastic polyps from the denominator, given their lack of malignant potential. Gallbladder stones were detected in 99 patients [14.4% (99/686)]. A vast majority of patients [628 (91.5%)] showed signs of chronic cholecystitis on histopathological examination (Table 1).
Comparison of the cholesterol polyp, adenoma, and adenoma canceration groups
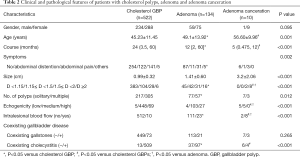
We compared the clinical and laboratory features among the cholesterol polyp group, adenoma group, and adenoma canceration group (Table 2).
Full table
The average age in the adenoma canceration group (56.60±9.96 years) was significantly greater than that in the adenoma (49.1±13.92 years) and the cholesterol polyp (45.23±11.45 years) groups (P=0.001). The median (25th percentile, 75th percentile) time between diagnosis and admission for surgery in the cholesterol polyp group [24 (3.5, 60) months] was significantly longer than that in the adenoma {12 [2, 60] months} and adenoma canceration [5 (0.475, 12) months] groups. The percentage of patients with symptoms in the cholesterol polyp group (51.4%) was significantly higher than that in the adenoma group (31.5%).
The mean diameter of lesions in the cholesterol polyp group (0.99±0.32 cm) was significantly smaller than that in the adenoma (1.41±0.60 cm) and adenoma canceration (3.2±2.06 cm) groups. The percentage of patients with a solitary polyp in the cholesterol polyp group [41.6% (217/522)] was higher than that in the adenoma group [57.5% (77/134)]. Eighty percent patients in the adenoma canceration group showed intralesional blood flow, which was significantly higher than that in the adenoma [17.2% (23/134)] and cholesterol polyp [1.9% (10/522)] groups. The percentage of patients with low echogenicity of lesions in the adenoma canceration group (50%) was significantly higher than that in the adenoma [3.0% (4/134)] and cholesterol polyp [1.0% (5/522)] groups.
Histopathology results showed that the percentage of patients with coexisting cholecystitis in the cholesterol polyp group [97.5% (509/522)] was significantly greater than that in the adenoma [72.4% (97/134)] and adenoma canceration [40% (4/10)] groups.
Predictive factors for adenomas
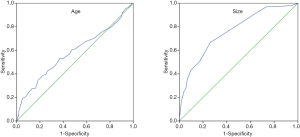
In order to identify to risk factors for adenomas, we compared the above results between 522 cholesterol GBPs and 134 adenomas. For detailed analysis, the age was divided into 2 categories using receiver-operator characteristic (ROC) curve analysis. A cut-off value of 49.5 years was associated with 53.0% sensitivity and 63.0% specificity for diagnosis of adenoma [area under the curve (AUC): 0.582]. Similarly, the maximal lesion diameter was also divided into 2 categories using ROC curve analysis. A cutoff value of 1.15 cm was associated with 66.7% sensitivity and 73.8% specificity for diagnosis of adenoma (AUC: 0.760) (Figure 2).
On univariate analysis, several clinical and radiological variables showed a significant association with adenoma; these included age >49.5 years (P=0.003), polyp size >1.15 cm (P<0.001), solitary polyp (P=0.008), intralesional blood flow (P<0.001), absence of symptoms (P=0.004), and absence of coexisting cholecystitis on histopathological examination (P<0.001). On multivariate analysis, polyp size (>1.15 cm), intralesional blood flow, and absence of histopathological signs of coexisting cholecystitis were independent predictors of adenoma [odd ratios (OR): 4.058 (P<0.001), 6.430 (P=0.001), and 11.762 (P<0.001), respectively; Table 3].

Full table
Discussion
Management of GBPs has been extensively investigated; however, most previous studies were focused on distinguishing between malignant and benign polyps (6,11,18). Previous reports defined all gallbladder carcinomas as malignant polyps; however, more than 80% gallbladder adenocarcinomas were shown to develop from dysplasia or carcinoma in situ, which were de novo cancers without preexisting adenomas (15). It is believed that adenoma may play a role in some cases of gallbladder cancer. The adenoma-carcinoma sequence for GBPs has been confirmed and 3–8% of adenomas are reported to have malignant potential. In this study, adenoma canceration was firstly defined as gallbladder adenocarcinoma with residual adenomatous tissue on histopathological examination. In our study, adenomas accounted for 19.5% (134/686) of all GBPs and the prevalence of malignant transformation among adenomas was 7.0% (10/144). As for the high malignant potential of adenoma and the unknown mechanism of canceration, it is important to distinguish adenoma accurately among clinically diagnosed GBPs.
The risk factors for malignant polyps in previous reports included advanced age, polyp size, sessile morphology of GBPs, presence of symptoms, associated stones, presence of DM, and rapid polyp growth (14,23,24). These reports defined all gallbladder carcinomas as malignant polyps, which we believe does not reflect the real possibility of malignant transformation of GBPs.
We found no sex-specific predilection of GBPs, which is consistent with the results of previous studies (22,25). Females were more susceptible to GBPs in this study (female:male ratio, 1.26:1). Many studies have demonstrated that older age is associated with increased risk of malignant polyps (11,24). The age threshold varied across studies, for example, 50 (22,25), 57 (11), or 65 years (23). The mean age in our study population was 46.06±12.12 years with a higher proportion of patients in the third, fourth, and fifth decades of life. The youngest patient in the adenoma canceration group was 43 years of age, while 80% patients were older than 60 years. Patients in the adenoma canceration group were significantly older than those in the adenoma and cholesterol polyp groups. Univariate analysis revealed a significant association between age >49.5 years and adenoma; however, on multivariate analysis, the association was not statistically significant.
It is not clear whether there is a relationship between the symptoms and risk of malignancy. Some studies found an association between symptoms and malignancy (11,26). However, others found no relationship between the two on multivariate analysis (27). In our study, 48.1% patients had symptoms; most common symptoms were right upper quadrant pain, abdominal distension, nausea, and dyspepsia. The cholesterol polyp group had a significantly higher percentage of symptomatic patients. On univariate analysis, asymptomatic polyp was a risk factor for adenoma; however, no association between symptoms and adenoma was observed on multivariate analysis. It is uncertain whether the polyps were the original cause of symptoms; symptoms alone cannot determine the nature of polyps; however, further laboratory or imaging evaluation is required in symptomatic patients.
There is insufficient data to suggest how long an adenoma is likely to be present before undergoing malignant change (14). Our results suggest that the malignant transformation of adenoma may occur over a relatively short time. The underlying mechanism of malignant transformation is unclear and further molecular research is urgently required.
With regards to concurrent gallbladder stone and malignant polyps, presence of cholelithiasis was reported as a risk factor for malignant transformation of GBP (11). However, other studies have found no definite association between concurrent cholelithiasis and malignant potential of GBPs (9). In this study, concurrent cholecystitis was significantly more common in the cholesterol polyp group. Both univariate analysis and multivariate analysis indicated coexisting cholecystitis as a risk factor for cholesterol polyps.
In several studies, polypoid lesion size ≥1 cm was an independent risk factor for malignant transformation (10,23,28). A large number of unnecessary cholecystectomies may be performed according to this criterion. Hence, some authors have put forward a higher threshold value for cholecystectomy (17,22). Adenomas have commonly been found in polyps <10 mm and even in those <5 mm in size (15,17,29). Consistent with a previous report, 13.4% (18/134) of adenomas in the present study were <10 mm in size and the smallest size was 5 mm. A small proportion of lesions <10 mm (and even <5 mm) in size are malignant (12,13,16). These studies indicate that size alone is not a reliable factor to guide treatment decision-making for GBPs. In our study, both univariate and multivariate analysis indicated that size >1.15 cm is a predictor of adenoma. Among the 428 polyps sized ≤1.15 cm, cholesterol lesions accounted for 89.5% (383/428) while only 10.5% (45/428) lesions were adenomas.
In a large study of 986 patients, only 6.6% of GBPs increased in size during the follow-up period. A rapid increase in size of polyp was shown to be associated with malignant transformation (30,31). Unfortunately, we could not investigate the growth of the polyps in this retrospective study. The follow-up mainly depends on clinicians’ judgement and there is no reliable data to support the ideal follow-up regime for polyps that are at a low risk of malignancy. In our experience, for patients with no indication for surgery, 3-monthly ultrasonography (US) should be recommended for lesions suspected to be neoplastic; otherwise 6-monthly US is recommended. The end-point for follow-up would be an increase in size or other indications for resection, or conversely a cessation of growth, reduction, or disappearance. We believe that rapid growth ≥3 mm in 6 months is an indication for surgical treatment.
US is the most commonly used modality for imaging of polypoid lesions of the gallbladder. Earlier studies suggest that US can identify the typical characteristics of neoplastic polyps (32). In some studies, endoscopic ultrasound (EUS) was found to be more accurate than conventional ultrasound (33). The diagnostic efficiency of contrast endoscopic ultrasound (CEUS) was shown to be superior to that of conventional ultrasound (34). However, EUS and CEUS are not suitable for routine clinical use, as these are invasive, expensive, and time-consuming investigations. Previous studies have tried to identify the predictable sonographic characteristics of premalignant and malignant polypoid lesions in the gallbladder, such as presence of intralesional blood flow, absence of hyperechoic spots, solitary polyp, marginal irregularity, sessile morphology, and loss of the gallbladder wall layer structure (32,35,36). In spite of vigorous efforts to standardize these US features, inter-observer variability is still the main limitation of its use for differential diagnosis. In polyps that exhibit these high-risk factors for neoplastic lesions, aggressive interventions such as EUS, CEUS, enhanced computed tomography, or magnetic resonance imaging are recommended.
Some studies suggested that a single polyp is more likely to be malignant (22,37-39), while other studies found that the risk of malignant transformation of solitary polyps was not higher than that of multiple polyps (3,16). Univariate analysis indicated solitary polyps as a risk factor for adenoma in our study; however, multivariate analysis showed no statistical significance. Moreover, the frequency of intralesional blood flow in adenoma canceration group was significantly higher than that in the adenoma and cholesterol polyp groups. Univariate analysis and multivariate analysis both showed intralesional blood flow as a risk factor for adenoma.
Some studies have found that the presence of gallstones is a risk factor for malignant transformation of GBPs (11,14,40), while contrary results have been reported by others (19,41). In our study, presence of gallstones was not a risk factor for adenoma and malignant lesions.
The relationship of GBPs with hyperlipidemia, DM, and obesity has been established previously (42). Studies have shown that metabolic syndrome contributes to the formation of cholesterol polyps in the gallbladder (43). In a previous study, serum cholesterol level in patients with cholesterol polyps tended to be higher; however, no significant between-group difference was observed in this respect (44). In our study too, no significant between-group difference was observed between the three groups.
The current guidelines recommend cholecystectomy when the GBP is greater than 1 cm in size, regardless of other factors. However, based on our findings, we propose a more accurate method for identification of neoplastic polyps. The size of GBP and intralesional blood flow are important criteria to determine the indication for surgery. Most patients with GBPs sized <1.15 cm and no signs of intralesional blood flow can be managed conservatively by dietary regulation and close follow-up. For GBPs that exhibit intralesional blood flow, cholecystectomy should be considered irrespective of the size owing to the high suspicion index for neoplastic polyp. Age and the number of polyps were not found to be important risk factors for neoplastic GBPs in this study.
Some limitations of our study need to be acknowledged. First, despite the large number of cases, this was a retrospective single-center study; a prospective multicenter study is required to validate our findings. Secondly, the number of patients in the adenoma canceration group was very low. It was difficult to further present the factors associated with adenoma canceration. Thirdly, US is a dynamic investigation and liable to inter-observer variability. Although we reviewed the reports as well as the imaging records carefully, the analysis of US results may have been influenced by subjectivity. Fourth, variables such as rate of growth of polyps, body mass index, dietary habits, family history, and occupation were not included in the analysis. Despite these limitations, we believe that our findings provide valuable insights for management of patients with GBPs.
In conclusion, preoperative diagnosis of malignant and premalignant lesions and their differentiation from benign lesions is challenging. We identified a few risk factors for neoplastic polyps: polyp size >1.15 cm, intralesional blood flow, and absence of coexisting cholecystitis. Malignant transformation of adenoma may occur over a relatively short period of time; however, the underlying mechanism of malignant change needs further molecular research.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the ethics committee of the China-Japan Friendship Hospital.
References
- Cairns V, Neal CP, Dennison AR, et al. Risk and Cost-effectiveness of Surveillance Followed by Cholecystectomy for Gallbladder Polyps. Arch Surg 2012;147:1078-83. [Crossref] [PubMed]
- Lin WR, Lin DY, Tai DI, et al. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol 2008;23:965-9. [Crossref] [PubMed]
- Marangoni G, Hakeem A, Toogood GJ, et al. Treatment and surveillance of polypoid lesions of the gallbladder in the United Kingdom. HPB (Oxford) 2012;14:435-40. [Crossref] [PubMed]
- Xu Q, Tao LY, Wu Q, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford) 2012;14:373-81. [Crossref] [PubMed]
- Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch Pathol 1970;90:423-32. [PubMed]
- Ito H, Hann LE, D'Angelica M, et al. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg 2009;208:570-5. [Crossref] [PubMed]
- Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg 1992;79:227-9. [Crossref] [PubMed]
- Konstantinidis IT, Bajpai S, Kambadakone AR, et al. Gallbladder lesions identified on ultrasound. Lessons from the last 10 years. J Gastrointest Surg 2012;16:549-53. [Crossref] [PubMed]
- Kwon W, Jang JY, Lee SE, et al. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci 2009;24:481-7. [Crossref] [PubMed]
- Lee KF, Wong J, Li JC, et al. Polypoid lesions of the gallbladder. Am J Surg 2004;188:186-90. [Crossref] [PubMed]
- Terzi C, Sokmen S, Seckin S, et al. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery 2000;127:622-7. [Crossref] [PubMed]
- Chou SC, Chen SC, Shyr YM, et al. Polypoid lesions of the gallbladder: analysis of 1204 patients with long-term follow-up. Surg Endosc 2017;31:2776-82. [Crossref] [PubMed]
- Kasle D, Rahnemai-Azar AA, Bibi S, et al. Carcinoma in situ in a 7 mm gallbladder polyp: Time to change current practice? World J Gastrointest Endosc 2015;7:912-5. [Crossref] [PubMed]
- Park JY, Hong SP, Kim YJ, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol 2009;24:219-22. [Crossref] [PubMed]
- Roa I, de Aretxabala X, Araya JC, et al. Preneoplastic lesions in gallbladder cancer. J Surg Oncol 2006;93:615-23. [Crossref] [PubMed]
- Shinkai H, Kimura W, Muto T. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg 1998;175:114-7. [Crossref] [PubMed]
- Kubota K, Bandai Y, Noie T, et al. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery 1995;117:481-7. [Crossref] [PubMed]
- Csendes A, Burgos AM, Csendes P, et al. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Ann Surg 2001;234:657-60. [Crossref] [PubMed]
- Park JK, Yoon YB, Kim YT, et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver 2008;2:88-94. [Crossref] [PubMed]
- Boulton RA, Adams DH. Gallbladder polyps: when to wait and when to act. Lancet 1997;349:817. [Crossref] [PubMed]
- Pandey M. Risk factors for gallbladder cancer: a reappraisal. Eur J Cancer Prev 2003;12:15-24. [Crossref] [PubMed]
- Bhatt NR, Gillis A, Smoothey CO, et al. Evidence based management of polyps of the gall bladder: A systematic review of the risk factors of malignancy. Surgeon 2016;14:278-86. [Crossref] [PubMed]
- Cha BH, Hwang JH, Lee SH, et al. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol 2011;17:2216-22. [Crossref] [PubMed]
- Shin SR, Lee JK, Lee KH, et al. Can the growth rate of a gallbladder polyp predict a neoplastic polyp? J Clin Gastroenterol 2009;43:865-8. [Crossref] [PubMed]
- Sarkut P, Kilicturgay S, Ozer A, et al. Gallbladder polyps: factors affecting surgical decision. World J Gastroenterol 2013;19:4526-30. [Crossref] [PubMed]
- Jones-Monahan KS, Gruenberg JC, Finger JE, et al. Isolated small gallbladder polyps: an indication for cholecystectomy in symptomatic patients. Am Surg 2000;66:716-9. [PubMed]
- Albores-Saavedra J, Chable-Montero F, Gonzalez-Romo MA, et al. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum Pathol 2012;43:1506-13. [Crossref] [PubMed]
- Sugiyama M, Xie XY, Atomi Y, et al. Differential diagnosis of small polypoid lesions of the gallbladder: the value of endoscopic ultrasonography. Ann Surg 1999;229:498-504. [Crossref] [PubMed]
- Zielinski MD, Atwell TD, Davis PW, et al. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg 2009;13:19-25. [Crossref] [PubMed]
- Koga A, Watanabe K, Fukuyama T, et al. Diagnosis and operative indications for polypoid lesions of the gallbladder. Arch Surg 1988;123:26-9. [Crossref] [PubMed]
- Moriguchi H, Tazawa J, Hayashi Y, et al. Natural history of polypoid lesions in the gall bladder. Gut 1996;39:860-2. [Crossref] [PubMed]
- Liu XS, Chen T, Gu LH, et al. Ultrasound-based scoring system for differential diagnosis of polypoid lesions of the gallbladder. J Gastroenterol Hepatol 2018;33:1295-9. [Crossref] [PubMed]
- Azuma T, Yoshikawa T, Araida T, et al. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg 2001;181:65-70. [Crossref] [PubMed]
- Zhang HP, Bai M, Gu JY, et al. Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion. World J Gastroenterol 2018;24:744-51. [Crossref] [PubMed]
- Sugiyama M, Atomi Y, Kuroda A, et al. Large cholesterol polyps of the gallbladder: diagnosis by means of US and endoscopic US. Radiology 1995;196:493-7. [Crossref] [PubMed]
- Sadamoto Y, Oda S, Tanaka M, et al. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy 2002;34:959-65. [Crossref] [PubMed]
- Elmasry M, Lindop D, Dunne DF, et al. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int J Surg 2016;33 Pt A:28-35.
- Lee H, Kim K, Park I, et al. Preoperative predictive factors for gallbladder cholesterol polyp diagnosed after laparoscopic cholecystectomy for polypoid lesions of gallbladder. Ann Hepatobiliary Pancreat Surg 2016;20:180-6. [Crossref] [PubMed]
- Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg 2000;87:414-7. [Crossref] [PubMed]
- Aldouri AQ, Malik HZ, Waytt J, et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol 2009;35:48-51. [Crossref] [PubMed]
- Choi SY, Kim TS, Kim HJ, et al. Is it necessary to perform prophylactic cholecystectomy for asymptomatic subjects with gallbladder polyps and gallstones? J Gastroenterol Hepatol 2010;25:1099-104. [Crossref] [PubMed]
- Lim SH, Kim DH, Park MJ, et al. Is Metabolic Syndrome One of the Risk Factors for Gallbladder Polyps Found by Ultrasonography during Health Screening? Gut Liver 2007;1:138-44. [Crossref] [PubMed]
- Yang HL, Kong L, Hou LL, et al. Analysis of risk factors for polypoid lesions of gallbladder among health examinees. World J Gastroenterol 2012;18:3015-9. [Crossref] [PubMed]
- Sandri L, Colecchia A, Larocca A, et al. Gallbladder cholesterol polyps and cholesterolosis. Minerva Gastroenterol Dietol 2003;49:217-24. [PubMed]


