
A novel scoring system for conversion and complication in laparoscopic liver resection
Introduction
Given its superiority of faster recovery, shorter length of stay, less blood loss, lower postoperative morbidity without compromising the survival, there has been an increasing trend toward laparoscopic liver resection (LLR) worldwide (1-4). With advances in laparoscopic equipment and technologies, indications of LLR have been extended to major and complicated hepatectomies, such as standard hemihepatectomy, extended hemihepatectomy, mesohepatectomy, and the associating liver partition and portal vein ligation for staged hepatectomy (5-7). Even for patients with colorectal cancer with liver metastases, LLR is an appropriate option offering benefits in short-term outcomes based on the Southampton Consensus (8).
Of note, according to the report from Second International Consensus Conference on laparoscopic hepatectomy, minor LLR is still in stage of reassessment while the major LLR remains in exploration stage (9). For a safe dissemination of LLR, several predictive models for conversion or scoring systems for degrees of difficulty have been established (10-16) (Table 1). Nevertheless, there is evidence that bias generated from the indirect relevance are sophisticated statistically, as the conversion and complication are not determined by surgical difficulty alone. In addition, thorough discussion of the incidence of conversion and complication is the key point during the informed consent process. A novel scoring system for conversion and complication is more efficient to make patients and their families understand the cost to benefit in LLR, which could prevent the subsequent dispute and compliant in case conversion and complication rate arise. And more experienced surgeon is recommended for skillful LLR in patients with high risk of complication or conversion.
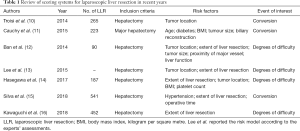
Full table
Therefore, such a scoring system for predicting conversion and complication has been long of interest. Based on our over ten-year experience of LLR, predictive models for conversion and complication were therefore conducted, which could facilitate the patient selection, avoiding excessive conversion and complication in clinical settings.
Methods
Patients and methods
This is a retrospective study approved by the Ethical Committees for Human Subjects at Sir Run Run Shaw Hospital (SRRSH), School of Medicine, Zhejiang University, China. In this study, those patients who underwent concomitant extrahepatic procedures (except cholecystectomy), vascular or biliary reconstruction, or two-staged hepatectomy were excluded. The indication for patients with laparoscopic hepatectomy was summarized as below: (I) American society of anesthesiologists (ASA) grade ≤ III; (II) Child-Pugh grade A or B; (III) symptomatic benign tumors or suspicious malignancies on preoperative image examinations; (IV) for malignancies, no distant metastasis was indicated, the number of lesions was ≤3 with each lesion less than ≤5 cm, restricted in hemihepatic lobe.
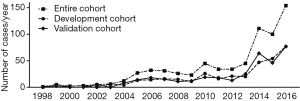
We started to perform LLR since August 1998. Initially, the laparoscopic peripheral wedge resection procedure was performed. With the accumulation of experience, the extent of resection was extended to major and complicated hepatectomy step by step. Between August 1998 and December 2016, 696 informed patients with pure LLR were reviewed eventually (Figure 1). Clinical information including demographic characteristics, pathological features, and surgical outcomes, was collected by independent investigators. Then, the entire cohort was randomly divided into development and validation cohorts with ratio of 1:1. In the development cohort (n=348), a multivariate logistic analysis with step-down elimination process was conducted. Based on the identified risk factors, predictive models for conversion and complication were established, respectively. Next, subgroup analysis of surgical outcomes followed by a comparison between the different models was performed.
Surgical technique
For laparoscopic hepatectomy, which was defined as pure laparoscopy. The operative details of laparoscopic hepatectomy have been previously described (3). In brief, after general anesthesia, a 10 mm camera trocar was inserted at the level of the umbilicus. Pneumoperitoneum was established using carbon dioxide to a pressure of 10–14 mmHg in general. Then three more ports were established with the navigation of laparoscopy: a 10 mm main port in the xiphoid for surgical manipulation and two 5 mm accessory ports on the right side of the abdomen. The position of trocars was altered appropriately according to the location of tumor. Ultrasound was used for intraoperative detection of lesion, if needed. The liver parenchymal transection was carried out using a Peng’s multifunctional operative dissector (Hangzhou Shuyou Medical Instrument Co., Ltd., Hangzhou, China) along with ischemia line. Meanwhile, the central venous pressure was maintained at 4–5 cmH2O. Vascular clips or polypropylene sutures were applied to control the divided biliary and vascular structures. The Pringle maneuver was carried out as a salvage measure for bleeding control. The specimen of the liver was retrieved and placed in a plastic bag. Drainage tubes were left if bleeding or bile leakage was suspected.
Definition of end points
Demographic characteristics, tumor features and surgical outcomes were collected retrospectively. The primary end points of this study were the feasibility (conversion) and safety (complication). While operative time, blood loss, the rate of transfusion and hepatic inflow occlusion with Pringle maneuver, and postoperative hospital stay were regarded as second end points. The extent of liver resection was defined by the Brisbane 2000 terminology (17). Among, the major hepatectomy contained equal or more than three segmentectomy according to the Couinaud segment. In this setting, anterolateral segment was defined as 2, 3, 4b, 5, 6 segment, posterosuperior segment was defined as 1, 4a, 7, 8 segment. For tumor located both in anterolateral and posterosuperior segments, the tumor position was defined as junction area. The severe of complication was classified according to the Clavien-Dindo classification (18). The 90-day mortality was defined as any death occurring within 90 days after liver resection.
Statistical analysis
The continuous variables were expressed as medians with ranges, while categorical data were presented as numbers with proportions. Correspondingly, the Mann-Whitney U test was used to compare continuous variables, while the Pearson Chi-square test or corrected Chi-square test, was used to compare categorical data as appropriate. The clinical variables of development cohort were analyzed with multiple logistic regression. Subsequently, risk models were established based on the risk factors with P values less than 0.05 in above-mentioned regression model. Concordance index (C-index) and Decision curve analysis (DCA) were calculated for the evaluation of discrimination by R software (version 3.3.3). Significance was considered when two-tailed P values were less than 0.05 by SPSS, version 22.0 for Windows (IBM Corporation, Armonk, NY, USA).
Results
Clinical characteristics
Totally, 696 patients who met the criterion were enrolled. All clinical variables, including demographic characteristics, pathological features and surgical outcomes, were summarized in Table 2. The rates of conversion in the development and validation cohorts were 8.3% and 10.3%, respectively. Additionally, compared with 12.6% of complication in the development cohort, a comparable rate of 12.9% was concluded in the validation cohort (n=348). The major complication (Clavien-Dindo grade ≥ III) was only 4.6% (32/696) in the entire cohort. With respect to the 90-day mortality, only one patient of validation cohort died at 45 days after hepatectomy.
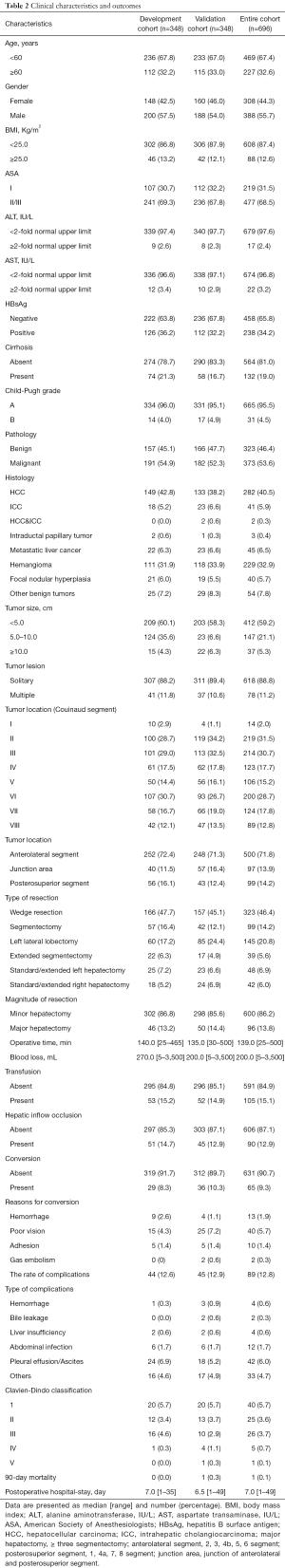
Full table
Establishment of risk models
The risk factors identified by logistic regression analyses for conversion, complication were presented in Table 3. In detail, the preoperative potential risk factors including age, gender, body mass index (BMI), ASA classification, liver cirrhosis, serum biochemical values, Child-Pugh grade, pathology, tumor size, lesion and location, magnitude of resection, were entered into the multiple logistic regression with step-down process. Alanine transaminase (ALT), ASA grade, and tumor location were related to conversion. While ALT, pathology, liver cirrhosis, tumor location, and magnitude of resection were associated with complication.
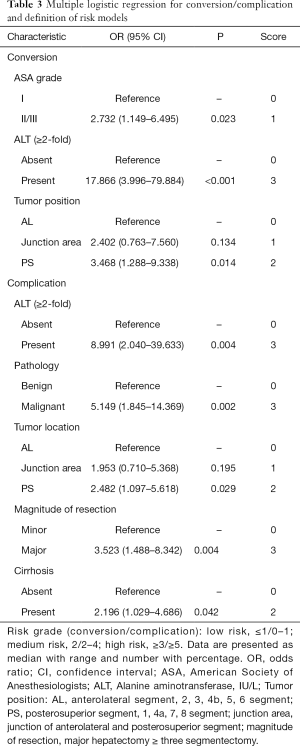
Full table
On this basis, the score in the present risk model (SRRSH risk model) was defined as follows. For conversion, ASA (I, score of ‘0’; II/III, score of ‘1’), ALT (<2 fold of normal upper limit, score of ‘0’; ≥2 fold of normal upper limit, score of ‘3’), tumor location (anterolateral segment, score of ‘0’; junction area, score of ‘1’; posterosuperior segment, score of ‘2’). For complication, ALT (<2 fold of normal upper limit, score of ‘0’; ≥2 fold of normal upper limit, score of ‘3’), pathology (benign, score of ‘0’; malignant, score of ‘3’), tumor location (anterolateral segment, score of ‘0’; junction area, score of ‘1’; posterosuperior segment, score of ‘2’), magnitude of resection (minor, score of ‘0’; major, score of ‘3’) and cirrhosis (absent, score of ‘0’; present, score of ‘2’). In total, the SRRSH risk models were classified into three levels: low risk, score ≤1; medium risk, score 2; and high risk, score ≥3 in conversion-derived risk model; while low risk, score ≤1; medium risk, score 2–4; and high risk, score ≥5 in complication-derived risk model, respectively (Table 3).
Surgical outcomes of subgroup analysis
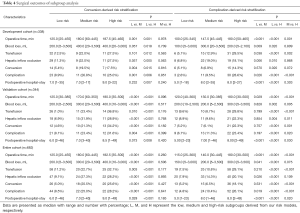
The results of subgroup analysis were presented in Table 4. In conversion-derived risk model, the conversion rate in the low risk groups (5.4%) was significantly lower than medium and high-risk groups (19.5%, P=0.004 and 17.5%, P=0.015). Compared with high risk group, the low risk group also showed superiority in other surgical outcomes, namely shorter operative time, less blood loss, lower rate of transfusion and complication, and the shorter postoperative hospital stay (P<0.05 for all). In validation cohort, a significant lower rate of conversion in low risk group was clarified in comparison of medium and high-risk groups (P<0.001 for both). Likewise, similar results were confirmed in the entire cohort, which indicated a better feasibility and safety of LLR in low risk patients.
Full table
For complication risk model. The rate of complication (2.6%) in the low risk group was significantly less than 9.5% of medium risk group and 28.6% of high-risk group in development cohort (P=0.026 and P<0.001). In validation cohort, despite no significant difference was conducted between low and medium risk groups in terms of complication, an apparently elevated rate of complication in high risk group was presented in comparison with low and medium risk group (23.4% vs. 11.3%, P=0.020 and 23.4% vs. 6.7%, P<0.001). Generally, a better surgical outcome in low risk group was demonstrated in all cohorts, compared with high risk group (P<0.05 for all). Taken together, high risk patients in complication-derived risk model presented an inferior consequence significantly compared with low risk patients.
Comparison between different risk models
Several risk models in regard to surgical difficulty has been conducted and primary comparison between different risk models has been performed (12-14,16,19,20). In this section, 10 patients in development cohort and 4 patients in validation cohort were excluded as the resection of caudate lobe was not involved in Iwate risk model (14). Therefore, 338 patients in development cohort, 344 patients in validation cohort, and 682 patients in entire cohort were used to assess the clinical application value of this novel model.
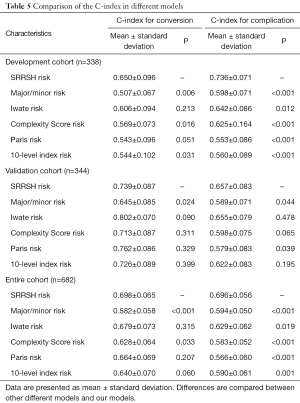
For discrimination, the C-index of these models was listed in Table 5. In development cohort, the C-index of SRRSH risk model for conversion was 0.650±0.096, which was significant higher than major/minor risk, Complexity Score risk and 10-level index risk models (0.507±0.067, P=0.006, 0.569±0.073, P=0.016 and 0.544±0.102, P=0.031). Contrary to expectations, this study did not find a significant difference of C-index between SRRSH risk and Complexity Score risk, 10-level index risk models in validation cohort. Totally, SRRSH risk model presented a better capacity of predicting conversion than major/minor risk and Complexity Score risk models. Additionally, compared with major/minor risk and Paris risk models, a higher C-index in SRRSH risk model was demonstrated in terms of complication both in development and validation cohorts. Similarly, an obvious superiority of SRRSH risk model in predicting complication was clarified in the entire cohort.
Full table
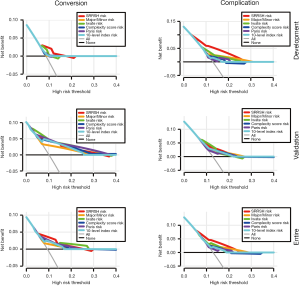
Given that the proposed SRRSH risk model demonstrated a superior predictive capacity in terms of the C-index, DCA was conducted to ascertain its applicability in clinic. In development cohort, SRRSH risk model had a better net benefit with a wider range of threshold probabilities for both conversion and complication. Furthermore, a non-inferior predictive capacity of SRRSH risk model was indicated in validation cohort. Likewise, our model presented a slight superiority in predicting the conversion compared with major/minor risk, Complexity Score risk, Paris risk, and 10-level index risk models in entire cohort. For complication, the DCA indicated the improved predictive performance at higher threshold probabilities of the SRRSH risk model, as well as its net benefit levels (Figure 2).
Discussion
Although laparoscopic hepatectomy is widely known to have superior short-term outcomes compared with open hepatectomy, the success of LLR cannot not be guaranteed even when it is performed by experienced surgeons (21-24). This is of major importance, since the goal of this study is to help a surgeon to know whether a patient is worth to undergo LLR or not. Previous classifications attempted to predict the likelihood of conversion and complication by grading the surgical difficulty of LLR (12-14,16). Dating back to 1956, Couinaud assessed the complexity of liver resection through defining three or more segmentectomy as major hepatectomy, which has been in use for over 50 years (13). Afterwards, the Complexity Score risk model based on an international survey of experts, 10-level index model, Iwate risk model and Paris risk model were conducted step by step. However, an effective and reliable scoring system to directly predict the rates of conversion and complication with LLR remains indispensable for patient selection and risk stratification.
In the present study, multiple logistic regression was performed to identify the risk factors for conversion and complication, respectively. Generally, the patients with high ASA grade representing an inferior general condition, are prone to occur disturbance of internal environment, and thereby a higher incidence of conversion with various reasons. Intriguingly, previous studies have confirmed that BMI is an unfavorable variable for feasibility and safety of LLR in western countries (14,25). But in our study, no association between a high BMI was indicated. Perhaps, the discrepancy between overweight and normal patients were quite small, which resulted in no significant difference in multiple logistic regression analysis. Additionally, the elevated ALT in serum, a classical injury marker, is demonstrated to be a positive correlation with severity of the damage in liver. Increased ALT always reflects a poor tolerance of stress and a damaged compensatory ability pathologically and physiologically, which could be an efficient predictive factor for both conversion and complication. Moreover, liver cirrhosis was another negatively prognostic element for complication. This might be responsible from two reasons. Initially, secondary portal hypertension and disordered coagulation function increase the possibility of bleeding, which is the ongoing hinder for widespread application of LLR. On the basis of fundamental research, the capacity of liver regeneration derived from hepatocyte deficiency is attenuated in diseased liver (e.g., fibrosis, cholestasis, steatosis, post-chemotherapy, etc.) (26-28). The delayed proliferation volumetrically and functionally aggravates the probability of complication.
In accordance with the present results, tumor location is another crucial variable to conversion (10,13,14). For instance, right posterior lobectomy is much harder than left lateral lobectomy, although both are minor hepatectomy. Compared with tumor belonging to anterolateral segments, the removal of tumor in posterosuperior segment presents a relatively higher morbidity because of poor visualization. Limited operating space and inadequate exposure give rise to increasing risk of conversion. For tumor in the junction area of anterolateral and posterosuperior segments, complicated hepatectomies such as hemihepatectomy or mesohepatectomy are always forced to perform, which presents a positive relationship with conversion. Theoretically, major resection contributes to higher surgical difficulty level and risk of conversion and complication. Insufficient future liver remnant (FLR) often raises the rate of complication, even the postoperative hepatic failure. In malignant tumor, liver resection is requisite to achieve a tumor-free margin. In fact, it is often difficult to achieve ideal tumor margins adjacent to the great vessels or hilum using laparoscopic techniques. Therefore, expanding the resection improves the likelihood of negative margins, which in turn contributes to a higher risk of postoperative liver failure or small-for-size syndrome because of an insufficient FLR.
Overall, compared with other models in regard to patient selection, some highlights of our models should be mentioned. First of all, both tumor factor and patients characteristics could have an effect on surgical outcomes. Some models were only focused on tumor factor but ignoring the patients’ general condition and liver function. In the present study, multiple analysis including tumor features and clinical characteristics was performed to establish the predictive models. The C-index and DCA represented a favorable discrimination, which was validated by extra internal cohort. Methodologically, the sophisticated method was quite novel and improved the credibility. The present study, to the best of our knowledge, was the first research establishing predicting models for feasibility and safety of LLR in terms of complication and conversion. Subsequent subgroup analysis indicated the SRRSH risk models were of great value in patient selection of LLR and could make patients and their families better understand the risk of LLR during the informed consent process.
However, the present study has some limitations that should be acknowledged. As the SRRSH risk models were derived from a single institution, an external validation trial was warranted definitely. Then, a majority of cases in our model were minor hepatectomy in normal liver; the generalization would be restrictive to some extent. Hence, more evidence on the benefits of laparoscopic approach for major resections or in diseased liver such cirrhosis are still needed to study whether the results would be justified in this setting. By accumulating LLR cases gradually, above-mentioned issues will be addressed as well as subgroup analysis with regard to major complication will be performed in our future work. Despite these limitations, we support that the SRRSH models present the remarkable capacities of predicting conversion, complication in LLR. And thereby, they could be a useful instrument to facilitate the patient selection for clinicians and communication with patients and their relatives during the informed consent process.
Acknowledgements
Funding: This study was supported by Department of Education of Zhejiang Province, China (grant No. Y201737942).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a retrospective study approved by the Ethical Committees for Human Subjects at Sir Run Run Shaw Hospital (SRRSH), School of Medicine, Zhejiang University, China.
References
- Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-7. [Crossref] [PubMed]
- Jackson NR, Hauch A, Hu T, et al. The Safety and Efficacy of Approaches to Liver Resection: A Meta-Analysis. JSLS 2015;19:e2014.00186.
- Cai X, Duan L, Wang Y, et al. Laparoscopic hepatectomy by curettage and aspiration: a report of 855 cases. Surg Endosc 2016;30:2904-13. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- de Santibañes E, Clavien PA. Playing Play-Doh to Prevent Postoperative Liver Failure. Ann Surg 2012;255:415-7. [Crossref] [PubMed]
- Narita M, Oussoultzoglou E, Ikai I, et al. Right Portal Vein Ligation Combined With In Situ Splitting Induces Rapid Left Lateral Liver Lobe Hypertrophy Enabling 2-Staged Extended Right Hepatic Resection in Small-for-Size Settings. Ann Surg 2012;256:e7-8. [Crossref] [PubMed]
- Cai X, Tong Y, Yu H, et al. The ALPPS in the Treatment of Hepatitis B-Related Hepatocellular Carcinoma with Cirrhosis: A Single-Center Study and Literature Review. Surg Innov 2017;24:358-64. [Crossref] [PubMed]
- Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg 2018;268:11-8. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Troisi RI, Montalti R, Van Limmen JG, et al. Risk factors and management of conversions to an open approach in laparoscopic liverresection: analysis of 265 consecutive cases. HPB (Oxford) 2014;16:75-82. [Crossref] [PubMed]
- Cauchy F, Fuks D, Nomi T, et al. Risk factors and consequences of conversion in laparoscopic major liver resection. Br J Surg 2015;102:785-95. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]
- Lee MK, Gao F, Strasberg SM. Perceived complexity of various liver resections: results of a survey of experts with development of a complexity score and classification. J Am Coll Surg 2015;220:64-9. [Crossref] [PubMed]
- Hasegawa Y, Wakabayashi G, Nitta H, et al. A novel model for prediction of pure laparoscopic liver resection surgical difficulty. Surg Endosc 2017;31:5356-63. [Crossref] [PubMed]
- Silva JP, Berger NG, Yin Z, et al. Minimally invasive hepatectomy conversions: an analysis of risk factors and outcomes. HPB (Oxford) 2018;20:132-9. [Crossref] [PubMed]
- Kawaguchi Y, Fuks D, Kokudo N, et al. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg 2018;267:13-7. [Crossref] [PubMed]
- Strasberg SM, Phillips C. Use and Dissemination of the Brisbane 2000 Nomenclature of Liver Anatomy and Resections. Ann Surg 2013;257:377-82. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Halls MC, Berardi G, Cipriani F, et al. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg 2018;105:1182-91. [Crossref] [PubMed]
- Lee SY, Chiow KH, Goh BK, et al. The challenge in determining difficulty of laparoscopic liver resection: are we there yet? Laparosc Surg 2018;2:23. [Crossref]
- Martínez-Cecilia D, Cipriani F, Vishal S, et al. Laparoscopic Versus Open Liver Resection for Colorectal Metastases in Elderly and Octogenarian Patients. Ann Surg 2017;265:1192-200. [Crossref] [PubMed]
- Okuno M, Goumard C, Mizuno T, et al. Operative and short-term oncologic outcomes of laparoscopic versus open liver resection for colorectal liver metastases located in the posterosuperior liver: a propensity score matching analysis. Surg Endosc 2018;32:1776-86. [Crossref] [PubMed]
- Hallet J, Sa Cunha A, Cherqui D, et al. Laparoscopic Compared to Open Repeat Hepatectomy for Colorectal Liver Metastases: a Multi-institutional Propensity-Matched Analysis of Short- and Long-Term Outcomes. World J Surg 2017;41:3189-98. [Crossref] [PubMed]
- Belli G, Limongelli P, Fantini C, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 2009;96:1041-8. [Crossref] [PubMed]
- de'Angelis N, Menahem B, Compagnon P, et al. Minor laparoscopic liver resection: toward 1-day surgery? Surg Endosc 2017;31:4458-65. [Crossref] [PubMed]
- Chaudhari P, Tian L, Deshmukh A, et al. Expression kinetics of hepatic progenitor markers in cellular models of human liver development recapitulating hepatocyte and biliary cell fate commitment. Exp Biol Med (Maywood) 2016;241:1653-62. [Crossref] [PubMed]
- Du Y, Wang J, Jia J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014;14:394-403. [Crossref] [PubMed]
- Español-Suñer R, Carpentier R, Van Hul N, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 2012;143:1564-75.e7. [Crossref] [PubMed]


