A novel role for polymeric immunoglobulin receptor in tumour development: beyond mucosal immunity and into hepatic cancer cell transformation
Chronic inflammation is well known as a significant driver of carcinogenesis in settings of human disease, including liver disease and hepatocellular carcinoma (HCC). Hepatitis B virus (HBV) is one of the major causes of HCC due to the oncogenic nature of the virus and the robust inflammatory response in the infected host. Fc receptors (FcR) are specialized receptors found on innate immune cells that recognize and bind to antigen-presenting antibodies, linking the innate and adaptive immune system to respond to circulating foreign bodies, such as viral DNA (1). Increased B-cell activation and antibody production in response to HBV infected liver will lead to increased FcR signalling on circulating innate immune cells and resident-macrophages (Kupffer cells), further enhancing the inflammatory response. Progression to chronic liver inflammation is associated with hyperactivity of B cell immunity, increasing the risk of cancer development due to constant infiltration of immune cells and accumulation of damaged hepatocytes. FcR activation has been previously implicated in carcinogenesis by creating a ‘pro-tumour microenvironment’ within inflammation-mediated damaged tissue through promotion of angiogenesis, epithelial mesenchymal transition (EMT), and increased cell survival (2). Of recent interest is the polymeric immunoglobulin receptor (pIgR), a member of the Fc receptor family, that is widely expressed on epithelial cells and is responsible for transcytosis of IgA/IgM at mucosal surfaces. Few studies have investigated the role of pIgR in cancer, although most have implicated pIgR to be downregulated in cancers of various epithelial origin (3). In this sense, the dysregulation of pIgR in mucosal membranes could limit the first line of defence for immunity against carcinogenic cells, and possibly contribute to malignant transformation.
In contrast, a previous study identified pIgR to be upregulated in colorectal cancers with liver metastasis and in HCC patients (4). Ai and colleagues showed that mice injected with Madin-Darby canine kidney (MDCK) cells or SMMC-7721 cells overexpressing pIgR had increased lung metastasis (4). The authors identified pIgR to activate Smad signalling and promote EMT in MDCK and SMCC-7721 cells, as demonstrated by increased migration and invasion activity (4). While these results elucidated how increased pIgR expression contributed to metastasis of epithelial tumours, it remained unknown how overexpression of pIgR may contribute to development of primary liver tumours. More recently this group was the first to show upregulated pIgR expression as a promoter of mesenchymal tumour formation (5). This study demonstrated both MDCK cells and SMMC-7721 cells overexpressing pIgR to have increased colony formation in vitro, including as anchorage-independent colonies, and was consistent with previous results (4). Furthermore, the injection of mice subcutaneously with these cancer cells resulted in significantly increased tumour growth, with a high mortality burden compared to control, and demonstrated the striking malignancy of this tumour subtype.
More notably Yue and colleagues are the first to identify a novel role for pIgR as part of a signalling cascade involved in HCC oncogenic transformation. Through a series of gene ablation experiments in vitro, the authors established the involvement of a number of down-stream effector kinases modulated by increased pIgR activation. In pIgR expressing MDCK and SMMC-7721 cells, pIgR formed a complex with an adapter protein, DAP12, and Src kinase member, Yes, to act as the primary signalling modulator. This complex recruited another tyrosine kinase, Syk, to activate GTPases Rac1 and Cdc42 which then activated MEK/ERK. Furthermore, pIgR was responsible for the activation of these downstream kinases, as demonstrated by Yes, Syk, and MEK/ERK phosphorylation, and increased expression of GTP-bound Rac1/Cdc42 (5).
The role of ERK in oncogenic transformation is myriad and is often stimulated by upstream regulators such as Ras/Raf, which are mutated in 20% of all human cancers (6). c-Myc is a well-known downstream target of ERK, and it induces the expression of cyclin D and cyclin-dependent kinase (CDK4; G1 phase), cyclin A (S phase), CDK1 required for mitosis, and CDK kinase inhibitors (CKI) such as p27Kip1 (7). ERK also activates ribosomal kinases (RSK) like p90RSK that can in turn activate CREB, and the proto-oncogenes c-Fos, Ets and c-Jun, to promote cell cycle promotion, stem cell renewal and differentiation (7-12). In addition, ERK can limit cell apoptosis by inactivating Bad/Bim, inhibitors of anti-apoptotic proteins Bcl-2 and Mcl-1, allowing Bcl-2/Mcl-1 to inhibit Bak/Bax and prevent mitochondrial outer membrane permeability (MOMP), release of cytochrome c and thus apoptosis (13). ERK also phosphorylates and inactivates caspase 9 to promote cell survival, and via CREB/Ets induce Bcl-2 and Bcl-XL transcription, to limit apoptosis (7). Therefore, pIgR stimulated activation of ERK may stimulate a large sequence of signalling events to promote tumour cell transformation, proliferation and survival (Figure 1).
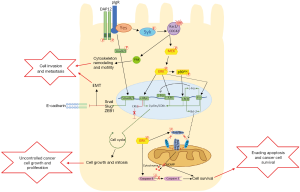
Additionally, Yue and colleagues demonstrated significant activation of Rho GTPase members Rac1 and Cdc42 (5), which activate p21-activate kinase (PAKs) to promote cytoskeleton reorganization for cell motility and invasion (14). Thus, increased activity of pIgR/Rac1/Cdc42 signalling may contribute to tumour cell invasion and metastasis, as demonstrated by the high malignancy of this tumour subtype in murine models (5). Previous work by these authors demonstrated pIgR to activate Smad2/3, increasing expression of Snail, Slug, and ZEB1 to repress E-cadherin expression and promote EMT (4). Therefore, overexpression of pIgR appears to not only contribute to continued cell cycle progression leading to cell growth, but also stimulates EMT and cell invasion, leading to a malignant and potentially metastatic tumour (Figure 1). This is consistent with other work that has observed pIgR expression to be correlated with colon cancer and liver metastasis (15).
This study also explored pIgR in a clinical setting of HCC. Yue and colleagues correlated pIgR positive tumours to be significantly correlated to tumours with active Yes/ERK. Additionally, pIgR/Yes positive tumours were associated with worse overall survival and disease-free survival in HCC patients, but only significantly in those who were further grouped as hepatitis B surface antigen (HBsAg) positive, or those with early-stage HCC (5). The presence of HBV infection may stimulate the expression of pIgR in an attempt to apprehend the circulating virus. Thus, the infected cells not only have increased expression of pIgR leading to aggressive tumour cell behaviour, but are also likely to simulate a large immune response and promote liver damage, further increasing the risk of HCC development. Other studies have identified increased expression of pIgR to be beneficial in preventing aggressive tumour formation, due to the contributions towards the immune response which may otherwise contribute to tumour growth (3). At this stage, it is unclear how the relative expression of pIgR contributes as a marker of prognosis, as it appears to be tissue dependent. The authors investigated the therapeutic potential of targeting the pIgR/Yes signalling cascade in an attempt to reduce tumour growth with dasatinib and AZD6244, inhibitors of Src kinases and MEK, respectively. Mice bearing MDCK xenograft tumours exhibited significantly decreased tumour volume and prolonged survival in both treatment groups, as expected since previous results showed significant increased activity of these kinases in this cell line. Mice bearing patient-derived pIgR+/Yes+ xenograft tumours showed greater reduction in tumour volume compared to the mice bearing pIgR-/Yes- patient tumours, although the response was considered non-significant (5). It is likely, that pIgR-/Yes- patient tumours did not respond well to treatment because of the activity of other oncogenic pathways. However, the increased sensitivity in the pIgR+/Yes+ tumours shows promise for specific patient-targeted therapy. Although applications as a primary treatment for HCC are unlikely, inhibiting this signalling cascade may provide value as a temporary therapeutic option for controlling tumour size to allow for resection or transplantation. Future investigations should explore the use of these inhibitors in clinical trials, and further characterization of this signalling cascade is required to confirm the role for pIgR in HCC development.
Acknowledgements
Funding: Cancer Council NSW Project Grant 1069733 (L Hebbard), Cancer Council Queensland Project Grant 1123436 (L Hebbard), James Cook Development Grant 2016 (L Hebbard), and a James Cook University Postgraduate Research Stipend Scholarship (B Dewdney).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pincetic A, Bournazos S, DiLillo DJ, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 2014;15:707-16. [Crossref] [PubMed]
- Andreu P, Johansson M, Affara NI, et al. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer cell 2010;17:121-34. [Crossref] [PubMed]
- Berntsson J, Lundgren S, Nodin B, et al. Expression and prognostic significance of the polymeric immunoglobulin receptor in epithelial ovarian cancer. J Ovarian Res 2014;7:26. [Crossref] [PubMed]
- Ai J, Tang Q, Wu Y, et al. The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma. J Natl Cancer Inst 2011;103:1696-712. [Crossref] [PubMed]
- Yue X, Ai J, Xu Y, et al. Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma. Hepatology 2017;65:1948-62. [Crossref] [PubMed]
- Torii S, Yamamoto T, Tsuchiya Y, et al. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci 2006;97:697-702. [Crossref] [PubMed]
- Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras//Raf//MEK//ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003;17:1263-93. [Crossref] [PubMed]
- Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. Embo J 1999;18:188-97. [Crossref] [PubMed]
- Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 1995;270:23589-97. [Crossref] [PubMed]
- Nagata D, Suzuki E, Nishimatsu H, et al. Transcriptional activation of the cyclin D1 gene is mediated by multiple cis-elements, including SP1 sites and a cAMP-responsive element in vascular endothelial cells. J Biol Chem 2001;276:662-9. [Crossref] [PubMed]
- Djaborkhel R, Tvrdik D, Eckschlager T, et al. Cyclin A down-regulation in TGFbeta1-arrested follicular lymphoma cells. Exp Cell Res 2000;261:250-9. [Crossref] [PubMed]
- Treisman R. The serum response element. Trends Biochem Sci 1992;17:423-6. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84. [Crossref] [PubMed]
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays: news and reviews in molecular, cellular and developmental biology 2007;29:356-70. [Crossref] [PubMed]
- Liu F, Ye P, Bi T, et al. COLORECTAL polymeric immunoglobulin receptor expression is correlated with hepatic metastasis and poor prognosis in colon carcinoma patients with hepatic metastasis. Hepatogastroenterology 2014;61:652-9. [PubMed]


