
Surgery for metastatic pancreatic neuroendocrine tumors: a narrative review
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are the second most common neoplasm of the pancreas comprising up to 10% of all pancreatic tumors (1). There has been a well-documented increase in the incidence of PanNETs (1,2). This in large part has been attributed to incidental findings from the increased use of cross-sectional imaging (1). PanNETs are derived from the endocrine islet cells of the pancreas and thus can secrete hormonally active substances. The vast majority of these tumors are non-functional, however, and in general display great heterogeneity in biological behaviors (3,4). Despite some of these tumors being indolent and having more favorable outcomes compared to the more common pancreatic ductal adenocarcinoma, up to 64% of patients with PanNETs present with metastatic disease and the 5-year survival of this group is around 57% (5-7). The liver is the most common site of distant metastasis with 50–80% of neuroendocrine tumors (NETs) eventually having hepatic involvement (8,9). Some studies suggest that DAXX/ATRX gene mutations, which are involved in the activation of the alternative lengthening of telomeres (ALT) pathway, confer higher risk of metastasis (10,11). Loss of function of these genes have been associated with increased tumor size, higher Ki67, and chromosomal instability. Other common genes mutated in PanNETs include MEN1, VHL, TSC, and genes within the PI3K/mTOR pathway (12).
Surgery remains the mainstay of treatment for PanNETs for localized tumors. There is increasing recognition that there may be a role for surgery in patients with distant metastasis as well (13-16). In this narrative review we will discuss the current surgical management of metastatic disease in patients with PanNETs. We hope to update what is currently known, uncover the controversies, and understand whether there is a benefit to performing surgery in this population. We present the following article in accordance with the Narrative Review reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-238/rc).
Methods
This narrative review was completed via a thorough literature search of the PubMed database using the search terms identified in Table 1. While many of the articles reviewed were published in the last 5–10 years, more dated articles from the 1990s were included both for historical context and because of the relatively limited scope of available literature on this topic. The first two authors primarily conducted the literature review and manuscript creation with the senior author providing expertise for any content disagreements and approving the overall discussion and perspective.
Table 1
| Items | Specification |
|---|---|
| Date of search | September 2021–June 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “Surgery pancreatic neuroendocrine tumor”, “metastatic neuroendocrine tumor”, and “liver debulking neuroendocrine tumor” |
| Timeframe | January 1990–June 2022 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Articles had to be available in free or full texts were excluded. Books, clinical trials, meta-analyses, randomized controlled trials, and reviews were considered. Only articles available in English language were considered |
| Selection process | All authors listed conducted the selection of articles independently to the needs of specific sections. There were instances of overlap as this specific subject area is relatively small. In general, each author reviewed the articles found by other authors for appropriateness and consensus was found if there were disagreements |
Classification and preoperative workup
Classification
PanNETs are first commonly classified either as functional (F-PanNETs) or non-functional (NF-PanNETs) (18,19). F-PanNETs only make up about 10–15% of PanNETs but can display a wide range of clinical syndromes based on the hormone it secretes (3,19). Insulinomas are the most common F-PanNET with gastrinomas being second. In MEN1 patients, of whom 50–75% will have PanNETs, gastrinomas are most common (15). Vasoactive intestinal peptide tumors (VIPomas), glucagonomas, and somatostatinomas are less common and make up the remaining F-PanNETs. Interestingly, there are also common anatomic locations for the various F-PanNETs. For example, insulinomas can be widely distributed throughout the pancreas whereas somatostatinomas are found commonly in the pancreatic head and VIPomas in the tail. Gastrinomas occur more commonly in the duodenum but also can be found in the pancreas (14). Knowledge of these common anatomic locations has implications for appropriate surgical treatment of primary tumors. Next, as shown in Tables 2,3, PanNETs are classified based on grade and stage (19,20). The World Health Organization’s (WHO) revised classification system released in 2017 has grades 1–3 based on mitotic index and Ki67 as well as differentiation of the tumor. This is important to note as high grade (G3) poorly differentiated tumors [referred to as pancreatic neuroendocrine carcinoma (PDNEC)] are treated with chemotherapy due to their aggressive nature, and surgery is generally not offered to these patients due to widely metastatic disease at onset and poor survival rates (21). High grade (G3) well-differentiated PanNETs however may be amendable to surgical removal of distant metastatic disease in certain cases if the tumor has proven to be biologically indolent.
Table 2
| Grade | Mitotic Index | Ki-67% | Differentiation |
|---|---|---|---|
| G1 PanNET | <2 | <3% | Well differentiated |
| G2 PanNET | 2–20 | 3–20% | Well differentiated |
| G3 PanNET | >20 | >20% | Well differentiated |
| G3 PanNEC | >20 | >20% | Poorly differentiated |
Adapted from Jeune et al. (20). WHO, World Health Organization; PanNET, pancreatic neuroendocrine tumor; PanNEC, pancreatic neuroendocrine carcinoma.
Table 3
| Stage | Details |
|---|---|
| T1 | Tumor limited to the pancreas <2 cm |
| T2 | Tumor limited to the pancreas >2 to <4 cm |
| T3 | Tumor limited to the pancreas >4 cm or invading duodenum or common bile duct |
| T4 | Tumor invades adjacent structures or vessels (CA or SMA) |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| M0 | No distant metastasis |
| M1 | – |
| M1a | Metastasis confined to the liver |
| M1b | Metastasis in at least one extrahepatic site |
| M1c | Both hepatic and extrahepatic metastasis |
AJCC 8th edition for PanNETs. Adapted from Jeune et al. (20). AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; CA, celiac artery; SMA, superior mesenteric artery; PanNETs, pancreatic neuroendocrine tumors.
Workup
Initial workup of PanNETs usually includes measurement of serum chromogranin A (CgA) levels (20). CgA is elevated in 60–80% of PanNETs and it has been shown to correlate with tumor burden and liver metastasis (20). CgA however is only about 65% specific and several other conditions have been shown to increase CgA levels including renal and liver failure, drugs like proton-pump inhibitors and antihistamines, as well as H. pylori infection, among others (15,20). The North American Neuroendocrine Tumor Society (NANETS) recommends its use as a marker for postoperative surveillance if preoperative levels are elevated (6). Neuron-specific enolase and pancreatic polypeptide have been explored as biomarkers but are also not very specific (20). Additional workup includes hormone level measurements for insulin, gastrin, glucagon, somatostatin, vasoactive intestinal peptide, or serotonin if a F-PanNET is suspected.
Imaging studies can be categorized as anatomical or functional. Anatomical imaging, including multiphase pancreatic protocol computed tomography (CT) and magnetic resolution imaging (MRI), are the first line anatomical imaging studies (6). MRI with EVOIST® is superior for smaller lesions and hepatic metastasis, but CT has the advantage of being easier to interpret and advantageous for small arterially enhancing primary tumors (6,22). 68Gallium and 64Cu-DOTATATE positron emission tomography (PET)/CT are functional imaging studies that have been used when traditional CT and MRI are indeterminate or when the primary tumor site is unknown (6,14). However, recent studies suggest that 68Gallium DOTATATE PET/CT changes inter- and intra-modality management in two thirds of metastatic patients and in almost a quarter of patients prior to surgery (23,24). It is worth noting that since most PDNEC and some well differentiated G3 PanNETs do not express somatostatin receptors, fluorodeoxyglucose (FDG) PET/CT rather than DOTATATE PET/CT should be employed to determine tumor burden (25). Therefore, functional imaging should be employed for staging and surveillance of PanNETs in our opinion.
NANETS guidelines also recommend the use of endoscopic ultrasound (EUS) specifically if there is a concern for multifocal tumors, as seen in MEN1 (6). Fine needle aspiration (FNA) should be performed only when the diagnosis is in question and/or to determine the tumor grade. However, it should be noted that a limitation of EUS/FNA for tumor grade is that these tumors display heterogeneity and there may be variation of the Ki-67 index within the tumor.
At our institution, every patient with a new diagnosis of a well differentiated metastatic PanNET will receive an MRI with EVOIST® and a DOTATATE PET/CT to assess liver tumor burden (LTB) and presence of extrahepatic and extra abdominal metastases.
Surgery for resectable metastases
Lymphadenectomy
Lymph node metastases in PanNETs may be more common in patients whose tumors are of larger size, located in the head of the pancreas, are higher grade, and show lymphovascular invasion (6). Unlike guidelines for colorectal or gastric adenocarcinoma, no formal recommendations have been made regarding the need for lymphadenectomy and an adequate number of harvested lymph nodes required in the setting of PanNET resection. While a National Cancer Database (NCDB) review by Gratian et al. identified no significant difference in survival between those who did or did not undergo lymphadenectomy as part of their PanNET resection (median of 8 lymph nodes examined), another review demonstrated that a lymph node yield between 11–15 nodes improved the likelihood of detecting nodal metastases (6,26). As such, NANETS guidelines suggest attempting to harvest at least 11 lymph nodes in a formal pancreas resection for PanNETs, but the long-term oncological impact of such a surgical step is unclear (6). In a metastatic setting, due to the high rate of recurrence after debulking, one could argue that a proper lymphadenectomy is not as important for staging, but rather for control of local disease recurrence and symptoms that may be associated with such tumors.
Resection of hepatic metastases
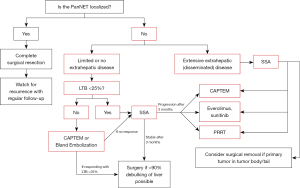
The liver is the most common site of metastases for NETs of any origin—with one national database review demonstrating the presence of hepatic tumors in up to 81% of metastatic cases (9). Because up to 64% of patients diagnosed with PanNETs present with distant metastases, surgeons frequently navigate management of pancreatic neuroendocrine liver metastases (NELMs) as well as extrahepatic lesions (6). Figure 1 illustrates the University of Chicago treatment paradigm for metastatic PanNETs.
Resectability guidelines
As the incidence of NETs has increased, focus has centered on the proper management of NELMs even though strict criteria defining resectability of these metastatic tumors have yet to be established. The most common cause of death in patients with NELMs is general hepatic failure due to overwhelming tumor burden. Therefore, oncologic principles that may pertain to other malignant tumors—obtaining negative margins, resecting the entire tumor burden, and excluding extra-hepatic disease—do not consistently apply to NELMs (27). Still, utmost importance is placed on the expectation and feasibility of resecting much of the tumor burden while maintaining a sufficient functional liver remnant. Many retrospective studies and expert guidelines have identified characteristics, such as different metastatic patterns, histological tumor grades 1 and 2, minimal extra-hepatic metastases, and the absence of carcinoid heart disease, that enhance the outcomes of surgical management for NELMs (28,29).
Benefits of surgical resection of metastases
PanNETs can cause general abdominal symptoms like pain and bloating or a constellation of signs pertaining to their respective hormonal functionality. Early in the era of surgical management for NELMs, studies focused on the resolution of symptoms as a primary outcome (30). A study from 2000 at Memorial Sloan Kettering reviewed management of NELMs, with those of pancreatic origin constituting the largest proportion of cases (42%) (31). The authors noted that 57% of patients initially treated with surgery had a primary complaint of hormonal symptoms or pain and all except one of those individuals had resolution of symptoms after surgical resection. In one of the largest single-institution studies, Sarmiento et al. included 170 patients with NELMs—52 of pancreatic origin—and likewise reported improvement or resolution of symptoms in 96% of cases after resection; although 59% experienced recurrent symptoms (32). Importantly, in this study, 5-year survival rates for both asymptomatic and symptomatic patients alike were improved compared to historically documented rates. Consequently, this review from Sarmiento et al. helped transition the focus of surgical management from symptoms to survival (33).
With the increasing use of surgical resection, survival outcomes data has expanded from single-institutional reviews to multi-institutional studies, national database reports, and meta-analyses. However, level I evidence is still lacking as no randomized control trials have been performed on this topic. To this point, the most recent PanNET guidelines from NANETS remarked that a consensus on surgical resection for pancreatic neuroendocrine liver metastases (pNELMs) was unable to be reached given the low level of evidence (6). In addition to the inherent selection bias when choosing patients for surgery that is prevalent in non-randomized studies, reviews often include patients with a variety of gastroenteropancreatic NETs, making it challenging to discern reliable results exclusive to those with PanNET origins.
Nevertheless, recent analyses of surgical resection, also referred to as hepatic debulking or cytoreduction, specifically for pNELMs have demonstrated promising associations between surgery and rates of survival. For example, Morgan et al. reported that their 5-year survival rate for pancreatic NELMs treated with surgical resection was 81%, with all deaths attributed to subsequent liver failure (33). Authors of a multi-institutional European study encompassing 166 patients with pancreatic NELMs compared those who underwent varying degrees of resection to those who underwent non-surgical treatment (34). They reported that the 91 patients in the resection group demonstrated significantly improved median overall survival (OS) from time of diagnosis (97 vs. 36 months, P<0.0001) as well as greater 5-year survival (76% vs. 36%). Although those in the operative group had significantly smaller median tumor size and lower rates of bilobar liver disease preoperatively compared to non-operative patients, a subsequent multivariate analysis revealed that surgical debulking remained an independent positive prognostic factor of OS. This multivariate model also accounted for negative prognostic factors like bilobar metastatic distribution and larger metastatic tumor size; a trait that also negatively impacted survival for those with tumors larger than 5 cm in the study from Morgan et al. (33,34).
Chawla et al. reviewed the National Cancer Database (NCDB) for patients with PanNETs in the head of the pancreas, comparing those that underwent pancreaticoduodenectomy for primary tumor resection vs. no primary tumor resection, with a further stratification for those that underwent metastasectomies (35). Patients who underwent both pancreatic and metastatic tumor resection had the longest median survival of 93.2 months, which dropped to 71.8 months if only pancreatic surgery was performed (P<0.001). Likewise, those who underwent hepatic metastases resection without a pancreatic operation had a longer median survival of 25.2 months compared to 15.2 months for those who had no surgery at all (P<0.001). Compared to those without metastasectomies, surgical resection of pNELMs was associated with significantly improved OS and was confirmed to be an independent positive predictor of survival on multivariate analysis.
Meta-analyses for NELMs exclusively of pancreatic origin are lacking, although a review in which PanNETs constituted a large proportion of cases demonstrated that resection of NELMs was associated with improved symptom relief and OS in the pooled studies (36). However, Lesurtel et al. performed a meta-analysis evaluating, not only the effectiveness of resection for NELMs, but also how it compared to other non-surgical treatment options like chemotherapy, embolization, or biotherapy (37). Pointedly, the authors highlighted both the selection bias in each study and the lack of a statistically significant association between treatment type and OS in the many individual multivariate analyses, leading to the conclusion that little evidence existed to pursue hepatic metastatic debulking over non-surgical options.
Ultimately, however, various reviews have linked NELMs resection to an improvement in symptoms and longer OS for patients with PanNETs, but this has not yielded a consensus on its overall benefits given the presence of selection bias, lack of randomized trials, and availability of other management options. Table 4 below summarizes the findings of several articles relating to resection of NELM (31-36,38-42).
Table 4
| Author | Year | Notesa | N (total/PanNET) | Patients undergoing surgical resection of NELM (%) | Primary tumor resectionb (%) | >70% Cytoreduction (%) | R0/R1c resection (%) | Improved symptoms postop (%) | Postop morbidity (%) | Overall recurrence (%) | Median RFS (mos) | 5-year RFS (%) | Median OS (mos) | 1-year OS (%) | 5-year OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meta-analysis | |||||||||||||||
| Yuan et al.d | 2016 | – | 1,124/1,124 | 47.5 | – | – | – | 10.9 (OR)* | – | – | – | – | 2.75 (WMD)* | – | 6.1 (OR)* |
| National database review | |||||||||||||||
| Chawla et al. | 2018 | NCDB | 4,038/4,038 | 9.1e | 4.5e | – | – | – | – | – | – | – | 93.2/71.8/25.2/15.2f* | – | – |
| Multi-institutional reviews | |||||||||||||||
| Aoki et al. | 2021 | – | 222/130 | 100 | 51.5g | – | 72.6 | – | 12.6 (90-day) | 83.4h | 14 | 13.4 | 113 | 91.8 | 70.2 |
| Zhang et al. | 2017 | – | 481/203 | 100 | – | – | 100 | – | – | 46.4 | – | 52.5 | – | – | 88.2i |
| Spolverato et al. | 2017 | – | 322/145 | 100 | – | – | 100 | – | – | 66.2j | 37 | 40.4 | – | – | – |
| Partelli et al. | 2015 | – | 166/166 | 55 | 55k | – | 19.8l | – | 46.2l | 76.5 | 33/15m* | – | 97/36m* | – | 76/36m* |
| Mayo et al. | 2010 | – | 339/134 | 100n | 84.7 | – | 74.1 | – | – | 46.6 | 15.2 | – | 125.1 | 92 | 74 |
| Single-institutional reviews | |||||||||||||||
| Morgan et al.o | 2018 | – | 44/37 | 100 | 47 | 100 | – | – | 18 | 43 | 11 | 4p | NR | – | 81 |
| Maxwell et al.o | 2016 | – | 108/28 | 100q | 96.4r | 64.3r | – | – | 64r | – | 19r | – | 126r | – | 76.1 |
| Sarmiento et al. | 2003 | – | 170/52 | 100 | – | 100 | – | 96 | 14 | – | 21 | 16 | 81 | – | 61 |
| Chamberlain et al. | 2000 | – | 85/42 | 40s | 42 | – | – | 100/94t | – | – | – | – | NR/NR/24u | 94/94/76u | 76/51/0u |
*, statistically significant; a, results are generally reported as units in header, unless otherwise notated. Studies with comparison groups have some results reported for multiple groups, check specific footnote; b, percent of patients who underwent prior or concomitant primary tumor resection at time of liver resection; c, R0 refers to a resection with negative margin. R1 refers to a resection with microscopically positive margin; d, results reported as odds ratio or weighted mean difference use surgical resection as the reference group compared to various forms of non-surgical management; e, 9.1% of all patients underwent surgical resection of hepatic metastases, 50% of whom also had resection of the primary tumor (4.5% of the total study sample). An additional 4.1% of all patients underwent surgical resection of the primary tumor only, which are not included in the “Primary tumor resection” reported percentage; f, results are reported for the 4 stratified groups in the study: primary tumor plus NELM resection/primary tumor resection only/NELM resection only/non-surgical; g, result reported applies only to those with PanNET origin (130 patients); h, result reported applies only to those with R0/R1 resection; I, result reported refers to NELM-specific survival, rather than overall survival; j, result reported applies only to those with PanNET origin (145 patients); k, result applies to the entire study population, meaning all patients who underwent surgery for NELM also had resection of the primary tumor; l, results reported apply to the percent of those undergoing surgery, not the entire study population; m, results reported as surgical cohort/non-surgical cohort; n, 10 patients underwent surgical ablation only of NELM, without any resection; o, studies arise from the same institution and may contain some overlapping patients in the PanNET group; p, estimated 5-year RFS; q, 4 PanNET patients underwent surgical ablation only of NELM, without any resection; r, results reported apply to only those with PanNET origin (28 patients); s, 40% of patients underwent surgical resection of NELM, 39% underwent hepatic artery embolization, 21% underwent medical treatment; t, result reported as surgical resection cohort/embolization cohort; no statistical comparison performed; u, results reported as surgical resection cohort/embolization cohort/medical management cohort; no statistical comparisons performed. PanNET, pancreatic neuroendocrine tumor; NELM, neuroendocrine liver metastases; R0, negative margin resection; R1, microscopically positive margin resection; RFS, recurrence free survival; mos, months; OS, overall survival; NCDB, National Cancer Database; NR, not reached.
Interestingly, newer data has suggested that surgical resection/debulking may play a role in improving efficacy of systemic therapies such as 177Lu-DOTATATE [Peptide Receptor Radionuclide Therapy (PRRT)]. For example, a sub analysis from the NETTER-1 trial suggested that progression-free survival (PFS) was decreased in patients with lesions >3 cm receiving PRRT, which may be due to decreased penetrance of PRRT in larger lesions (43). Surgical resection/debulking of larger lesions could therefore potentially improve efficacy of PRRT. A recent study also demonstrated that primary tumor resection improves survival in PanNET patients receiving PRRT when compared to those patients who did not undergo surgical removal of their primary tumors (44).
The exact role of surgical tumor debulking and its influence on systemic therapies therefore need to be studied further.
Recurrence of disease
One consistency throughout most studies covering surgical resection for NELMs is the high rate of metastatic recurrence observed. In their pancreatic NELMs specific review, Partelli et al. document a recurrence rate of 76.5% after surgical resection while a recent multi-institutional study from Japan noted only a 13.4% 5-year recurrence-free survival rate in their surgically treated patients, 59% of which had PanNET primaries (34,38).
A couple of multi-institutional studies have evaluated risk factors for recurrence and subsequent management following initial NELMs resection. PanNET primaries constituted 42% and 45% of cases and the total rate of recurrence in the entire cohort was 46% and 65%, respectively (39,40). In both reviews, the diagnosis of early recurrence (within 3 years) occurred in 71–72% of cases and was associated with worse disease-specific or OS. Certain factors like pancreatic primary tumors (vs. gastrointestinal tumors) and positive margin/R1 resections (vs. negative margins/R0) were found to have inconsistent associations with early recurrence between the two studies. Importantly, both groups emphasized improved survival outcomes for patients who underwent a curative procedure (including re-resection) for their recurrent metastases. While Zhang et al. noted a 28% increase in 10-year disease-specific survival for those with early recurrence who underwent curative treatment (P=0.028), Spolverato et al. documented 60% 10-year OS rates for those undergoing re-resection, which was significantly higher than those undergoing non-curative therapy (39,40).
Despite possible benefits of initial surgical resection for NELMs, recurrence of disease is unfortunately an expectation rather than an outlier. Yet, even surgical management of recurrent metastatic disease may play an important role by “resetting the clock” to minimize the burden of liver disease, and thereby, delay the onset of liver failure, enhance patients’ quality of life, and amplify benefits from systemic therapies (45).
Debulking threshold, resection margins, and parenchymal sparing surgical techniques
Although there currently remains a lack of expert consensus on whether to perform surgical resection of pNELMs, those centers who routinely perform these operations have aimed to identify what percentage of liver tumor debulking would be ideal to preserve the potential survival benefit associated with these procedures.
Historically, resection of 90% of grossly visible disease was deemed a sufficient debulking threshold to achieve positive results, which stemmed from the report by McEntee et al. on symptomatic resolution after resection (46). When Sarmiento et al. shifted the focus to survival metrics, they described their “non-curative” resections as those removing at least 90% of the tumor burden, and thereby, the 90% threshold became associated with improved survival as well (32,33). In the past decade, this debulking threshold has been challenged. Maxwell et al. explored results after hepatic tumor resections of different proportions for a group of 28 pNELMs (42). Notably, patients who had >70% of hepatic metastatic disease resected had statistically significant improvements in OS and PFS compared to those who underwent <70% resection. Meanwhile, a tumor resection >90% demonstrated improved PFS but no significant difference in OS compared to those with <90% resected. This report was followed by the single institution study from Morgan et al. who analyzed 37 patients with pNELMs who underwent tumor burden resection of >70% (8 patients), >90% (12 patients), or 100% (24 patients) (33). The authors reported no significant differences in either PFS or OS when comparing patients from these three different resection threshold groups.
Some earlier studies noted that only 9–25% of patients with NELMs were chosen for resection when a higher debulking threshold was utilized, while 76% of those with NELMs underwent metastatic resection in the study by Maxwell et al. (42). Given the positive outcomes associated with the 70% threshold, some surgeons have pushed for a larger number of patients to be deemed eligible for NELMs debulking, which would allow for more patients to reap the potential survival and symptomatic benefits that are linked to surgical resection.
While the debulking threshold is typically an estimated proportion of gross disease that is resected, margin status can serve as a more objective measurement determined from operative pathology and may carry similarly important influence on postoperative outcomes. Margin status is often divided into three designations: negative margin (R0), microscopically positive margin (R1), and macroscopically positive margin (R2). Mayo et al., who reported an overall 94% 5-year recurrence rate for patients undergoing resection of NELMs of all origins, noted that margin status did not statistically impact the rate of recurrence (41). For OS, an R2 resection carried no influence for those with nonfunctional tumors, but, contrastingly, an R0 or R1 resection in those with functioning NELMs showed statistically improved survival compared to those with an R2 resection. Likewise, margin status was analyzed in the multi-institutional European study from Partelli et al. that demonstrated improved OS for those undergoing pancreatic NELMs surgery (34). When categorizing the surgical group into those with R0/R1 resections vs. R2 resections, the authors demonstrated improved median OS in the R0/R1 cohort compared to both those with R2 resection and no resection at all (97 vs. 89 vs. 36 months, respectively; P=0.001). While microscopically positive margins were grouped with negative margins in this study, a separate study by Zhang et al. showed that R1 resections were linked to early intra-hepatic tumor recurrence compared to R0 resections (39).
Because NELMs can spread throughout the liver and mortality is often secondary to liver failure, techniques that allow for an adequate proportion of disease resection while maintaining a sufficient volume of healthy liver parenchyma are crucial. Parenchymal sparing surgical techniques, which include liver wedge resections/enucleations and (radiofrequency or microwave) tumor ablations, have expanded in use as reviews demonstrated acceptable outcomes with the 70% debulking threshold and positive margins. For example, Maxwell et al. reported that 14% of pancreatic NELM patients had only an ablation procedure, 57% underwent wedge resections or enucleations with concurrent ablations, while 3.6% had some form of formal resection (42). Authors of national database reviews report a majority of patients have anywhere from 1 to 5 partial/wedge liver resections with others even surpassing ten individual resections (47,48). In one such review by Scoville et al., concurrent tumor ablation actually decreased postoperative morbidity in a univariate analysis, although this impact was diminished in the multivariate analysis (47).
Naturally, the greater the LTB, the more difficult it can be to reach a sufficient resection threshold. A follow up to the study by Maxwell et al., concluded that there was a significantly smaller chance of achieving at least 70% hepatic tumor cytoreduction when LTB exceeded either 45% of the hepatic volume or 30 total lesions (42,49). Because most patients chosen for surgical resection throughout these studies carry a much smaller tumor burden than this, it is apparent that surgeons rightfully and heavily weigh the feasibility of achieving an adequate resection when offering surgical management for pancreatic NELMs. Ultimately, a 70% debulking threshold can make surgery more feasible for many patients, but further clarification of how both this clinical cutoff and margin positivity contribute to patient outcomes is paramount. In our center, we will generally aim for a 90% debulking threshold which we find is most consistently achieved with an LTB <25%.
Complications of liver debulking and primary tumor resection
As with hepatic resections for any indication, postoperative complications in the setting of pancreatic NELMs can include wound infections, hemorrhage, bile leaks, and hepatic abscesses (27). In some of the single and multi-institutional reviews, overall morbidity following hepatic debulking has exceeded 50% (34,42). However, as much as 70% of the complications are categorized as minor and grade 1 or 2, which includes superficial wound infections. Postoperative pancreatic fistula was the most common intra-abdominal complication in the study by Partelli et al., a result of the primary tumor resection rather than the hepatic procedures (34). Scott et al. found similar rates of overall complications in their NELMs cohort with combined pancreatic and small intestine primaries (49). They reported bleeding and intra-abdominal infection as the most common major complications. Furthermore, they stratified patients by the number of hepatic lesions resected (1–5, 6–10, >10) and discovered no statistically significant difference in postoperative complication rates between the groups.
Recent reviews of the National Surgical Quality Improvement Program (NSQIP) database analyzed hundreds of patients undergoing hepatic cytoreductive surgery for NELMs from any primary site. Reported 30-day mortality rates were approximately 1.5%, which is similar to the 2% mortality rate depicted in the pancreatic-exclusive study of Partelli et al. (34,47,48). In the NSQIP database, complications arose in 25–30% of patients, with major complications accounting for 15%. One review emphasized that an increasing number of tumor resections per patient was associated with a higher likelihood of experiencing postoperative morbidity (48). The other NSQIP review noted that concurrent ablation procedures had a protective link with developing morbidity and that simultaneous primary tumor resections were not associated with heightened morbidity on multivariate analysis (47). However, for pancreatic primary NETs specifically, concerns have arisen over whether both resection of the primary tumor and hepatic metastases may increase the risk of developing hepatic abscesses.
Simultaneous resection of primary tumor and metastatic disease
Whether the primary PanNET should be removed in conjunction with NELMs debulking has largely been considered in the context of complication risks and OS rates. Naturally, these two variables may be influenced by the location of the tumor within the pancreas. While analyzing patients with various pancreatic head tumors and liver metastases of which 35% were NETs, De Jong et al. reported statistically significant increased rates of general complications and hepatic abscesses in those who underwent a pancreaticoduodenectomy followed, later, by a separate liver-directed therapy (staged approach) vs. those with simultaneous treatment of both sites (50). The authors postulated that hepatic abscess formation may be limited by performing ablations or resections of hepatic parenchyma prior to introducing the potential for bacterial contamination from the biliary-enteric anastomosis of the pancreaticoduodenectomy. A multi-institutional neuroendocrine review from Japan included large numbers of concomitant pancreas and NELMs resections, including 16 pancreaticoduodenectomies, with reportedly acceptable 12–13% 90-day morbidity rates (38). The recent NANETS guidelines reiterated the safe outcomes demonstrated with simultaneous pancreatic tumor and NELMs resection and remarked that resection of pancreatic head tumors should be avoided prior to NELMs directed therapy given the concern for hepatic abscess development (6).
Studies have portrayed a mixed picture for survival outcomes. In the PanNET exclusive cohort described by Morgan et al., neither the completion of a primary tumor resection nor the timing of such resection with regards to NELMs debulking was correlated with survival (33). A large multi-institutional review assessed the effect of primary tumor resection on patients with non-functional gastroenteropancreatic NELMs, and the authors concluded that the status of primary tumor resection had no statistically significant impact on OS (51). Importantly, this statistical equivalency was demonstrated after propensity score matching was performed for the presence of pre-operative tumor characteristic imbalances. Meanwhile, authors of the NCDB review for NETs in the pancreatic head reported that, not only was metastatic tumor resection associated with improved survival, but also that primary tumor resection via a pancreaticoduodenectomy was independently prognostic of improved survival on Cox proportional hazards model (35). Although resection of the primary tumor can theoretically improve survival by lessening the overall tumor burden and enhancing effectiveness of non-surgical treatments, it is important to recognize that many patients with NELMs ultimately succumb to liver failure—rather than the primary tumor itself—and additional procedures like pancreaticoduodenectomies carry inherent mortality risks. Consequently, even an unresectable primary tumor may not preclude patients from undergoing surgical management of liver metastases.
In our center, we usually perform liver debulking and distal pancreatectomies for pancreatic body/tail tumors during the same operation. For pancreatic head tumors, we tend to first debulk the liver and then perform a pancreaticoduodenectomy later, usually 3–6 months after initial debulking surgery (unless the LTB is very low).
Resection of extrahepatic metastases
Beyond the liver, common locations for PanNET metastases include bones, distant and retroperitoneal lymph nodes, and the peritoneum (9,52). Because most patients with extrahepatic disease also have hepatic disease that can lead to hepatic failure, the presence of extrahepatic tumor deposits should not be an absolute contraindication to pursuing surgical management of hepatic metastases nor primary tumors—a sentiment echoed in the NANETS PanNET guidelines (6). Despite this, many reviews have found the presence of extrahepatic metastases to be a negative prognostic factor on survival for those with NELMs (37,41,51). Yet, median survival for those with extrahepatic disease has reached as long as 85 months, which may be an indication to proceed with resection of hepatic or other metastases when able (41). At our institution, we will proceed with hepatic and extrahepatic abdominal debulking of tumors, if the majority of the tumor is within the abdomen and if the debulking threshold of >90% can be achieved.
Peritoneal carcinomatosis is one manifestation of extrahepatic disease that may be amenable to resection itself. Although more commonly encountered in small bowel or appendiceal NETs rather than PanNETs, peritoneal neuroendocrine deposits that yield a Peritoneal Carcinomatosis Index (PCI) score under twenty have been estimated as potentially resectable. Per de Mestier et al., indications for cytoreduction of peritoneal metastases include intestinal obstruction, intra-abdominal fibrosis, and the potential for shifting to liver-specific treatments in those with unresectable hepatic metastases (52). Although hyperthermic intraperitoneal chemotherapy (HIPEC) is used in conjunction with cytoreduction for peritoneal deposits from other malignancies, most concur there is little evidence to support its use in NET pathology (52,53). Overall, meaningful outcomes data is lacking in the setting of peritoneal cytoreduction.
Surgical resection in the setting of unresectable distant metastases
Resection of primary only
Resection of the primary PanNET only, in the setting of unresectable metastasis, is thought to have several benefits. Resection of the primary only may improve survival, control symptoms from functional tumors, and prevent life-threating complications like bleeding, obstructive jaundice and pancreatitis, as well as gastroduodenal obstructions (6,54). European Neuroendocrine Tumors Society (ENETS) consensus guidelines only recommend resections in G1 and G2 tumors to prevent the aforementioned complications and to allow treatment of liver metastases (29). Although NANETS did not achieve consensus on this topic, they recommend the decision to resect primary tumors in this setting should be based on patient tumor functional status, age, comorbidities, obstructive symptoms, and location of the tumor due to increased morbidity of pancreatic surgery from pancreaticoduodenectomy (6). Several studies and meta-analyses have shown a survival benefit to primary tumor resection in the presence of unresectable liver metastases (44,55-58). Bertani et al., in 2017, prospectively showed an OS of 111 months in patients undergoing distal pancreatectomy vs. 52 months in patients who did not undergo resection (56). Zhou et al. in their meta-analysis demonstrated a mean OS of 36–137 vs. 13.2–65 months in resected cohorts and unresected cohorts respectively (55). Keutgen et al., looking at NF-PanNETs, showed median survival rates of 65 vs. 10 months in patients with primary tumor resection and without resection respectively (13). In another study reviewing the NCDB, Tierney and others found OS of 63.6 months in the resected cohort vs. 14.2 months in those without resection (59). Bertani in 2016 and Kaemmerer also looked at resection of the primary tumor alone in conjunction with PRRT. Both found significantly improved OS and PFS in resected patients treated with PRRT vs. those undergoing PRRT alone (44,58). Resection of primary tumors may increase the efficacy of PRRT perhaps by increasing the concentration and uptake of this radiation therapy with smaller and fewer tumors (58). Again, many of these studies are retrospective in nature and with a bias toward resection of younger, higher functional status patients with lower grade and more distal pancreatic tumors. Also noteworthy is none of these studies specifically assessed symptom control or quality of life, which are important theoretical benefits of primary tumor resection.
Liver transplantation
Orthotopic liver transplantation (OLT) is rarely used in patients with metastatic PanNETs but may be an acceptable option for selected patients with unresectable NELM. PanNETs account for the highest proportion of OLTs for NETs ranging from 44–53% of cases (60). Absolute criteria and indications are lacking, but guidelines like the Milan criteria for NETs and those from the Organ Procurement and Transplantation Network recommend restricting liver transplants to those younger than 60 years of age with histological grade 1 or 2 tumors, a primary tumor drained by the portovenous system, no tumor progression or recurrence after resection for 6 months, and an extensive hepatic burden that is bilobar but comprises <50% of the liver parenchyma (61). However, many of these suggestions are derived from evidence that is restricted to retrospective institutional and database reviews, which can be conflicting. For example, reviews of the United Network for Organ Sharing database demonstrated improved 5-year survival for patients that underwent OLT after a median 67-day wait time vs. those who underwent earlier surgery (62). But review of the European Liver Transplant Registry did not show an association between prognosis and time from diagnosis to OLT (60). Theoretically, a longer suggested wait time, like 6 months, prior to OLT can allow for preoperative optimization in the setting of prior primary tumor resection as well as disease progression monitoring that could forecast worse post-transplant outcomes (60). Resection of the primary tumor prior to OLT is common, and simultaneous primary resection and OLT is generally avoided given demonstrated poorer outcomes (60). Meanwhile, known extrahepatic metastases, unless resectable themselves, are often considered a contraindication to OLT.
As far as outcomes of OLT for unresectable NELMs, recurrence of disease is still a concern. Recurrences ranged from 13–57% after transplant, with time to recurrence varying widely among several studies (61,63). For patients undergoing OLT, OS has demonstrated promising trends in single institutional, national database, and systematic reviews alike, with quoted 1-year and 3-year survival rates reaching 81% and 65%, respectively (61,64). One prospective single institution review from Mazzaferro et al., who used the Milan criteria to select patients for transplantation, recorded 5-year OS rates as high as 97% in their patients undergoing transplant vs. 51% in those who did not undergo OLT but still met the criteria for selection (63). Notably, the two cohorts of patients differed significantly in age, tumor T stage, and rate of preoperative locoregional NELMs management, thereby influencing the interpretation of outcomes—a limitation present throughout the spectrum of studies reviewing surgical management for NELMs.
Conclusions
PanNETs are a group of heterogenous tumors that are becoming more common. Surgery remains the best treatment for localized tumors and has an important role to play in metastatic disease as well. Surgical debulking in selected patients with metastatic PanNET may confer a survival benefit, improve symptoms, and allow systemic therapies to be more efficacious. However, there remains lack of randomized prospective studies on the benefit of surgical debulking. In addition, it remains unclear where surgical debulking fits in the current treatment sequence paradigm for these tumors. These areas therefore present an opportunity for future investigation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-238/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-238/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Klimstra DS, Beltran H, Lilenbaum R, et al. The spectrum of neuroendocrine tumors: histologic classification, unique features and areas of overlap. Am Soc Clin Oncol Educ Book 2015;92-103. [Crossref] [PubMed]
- Ehehalt F, Saeger HD, Schmidt CM, et al. Neuroendocrine tumors of the pancreas. Oncologist 2009;14:456-67. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:1-33. [Crossref] [PubMed]
- Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol 2011;29:3044-9. [Crossref] [PubMed]
- Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist K, et al. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer 2016;139:2679-86. [Crossref] [PubMed]
- Pea A, Yu J, Marchionni L, et al. Genetic Analysis of Small Well-differentiated Pancreatic Neuroendocrine Tumors Identifies Subgroups With Differing Risks of Liver Metastases. Ann Surg 2020;271:566-73. [Crossref] [PubMed]
- Cives M, Partelli S, Palmirotta R, et al. DAXX mutations as potential genomic markers of malignant evolution in small nonfunctioning pancreatic neuroendocrine tumors. Sci Rep 2019;9:18614. [Crossref] [PubMed]
- Mafficini A, Scarpa A. Genomic landscape of pancreatic neuroendocrine tumours: the International Cancer Genome Consortium. J Endocrinol 2018;236:R161-7. [Crossref] [PubMed]
- Keutgen XM, Nilubol N, Glanville J, et al. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery 2016;159:311-8. [Crossref] [PubMed]
- Vaghaiwalla T, Keutgen XM. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am 2020;29:243-52. [Crossref] [PubMed]
- Johnston ME 2nd, Carter MM, Wilson GC, et al. Surgical management of primary pancreatic neuroendocrine tumors. J Gastrointest Oncol 2020;11:578-89. [Crossref] [PubMed]
- Keutgen XM, Schadde E, Pommier RF, et al. Metastatic neuroendocrine tumors of the gastrointestinal tract and pancreas: A surgeon’s plea to centering attention on the liver. Vol. 45, Seminars in Oncology. W.B. Saunders; 2018:232-5.
- Guidelines for Authors. Hepatobiliary Surgery and Nutrition. Available online: https://hbsn.amegroups.com/pages/view/guidelines-for-authors#content-2-2-3
- Cloyd JM, Poultsides GA. The Landmark Series: Pancreatic Neuroendocrine Tumors. Ann Surg Oncol 2021;28:1039-49. [Crossref] [PubMed]
- Liu JB, Baker MS. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Clin North Am 2016;96:1447-68. [Crossref] [PubMed]
- Jeune F, Taibi A, Gaujoux S. Update on the Surgical Treatment of Pancreatic Neuroendocrine Tumors. Scand J Surg 2020;109:42-52. [Crossref] [PubMed]
- Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015;39:683-90. [Crossref] [PubMed]
- Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol 2015;28:193-202. [PubMed]
- Tierney JF, Kosche C, Schadde E, et al. (68)Gallium-DOTATATE positron emission tomography-computed tomography (PET CT) changes management in a majority of patients with neuroendocrine tumors. Surgery 2019;165:178-85. [Crossref] [PubMed]
- Babazadeh NT, Schlund DJ, Cornelius T, et al. Should (68)Ga-DOTATATE PET/CT be Performed Routinely in Patients with Neuroendocrine Tumors Before Surgical Resection? World J Surg 2020;44:604-11. [Crossref] [PubMed]
- Taskin OC, Clarke CN, Erkan M, et al. Pancreatic neuroendocrine neoplasms: current state and ongoing controversies on terminology, classification and prognostication. J Gastrointest Oncol 2020;11:548-58. [Crossref] [PubMed]
- Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21:3515-21. [Crossref] [PubMed]
- Farley HA, Pommier RF. Treatment of Neuroendocrine Liver Metastases. Surg Oncol Clin N Am 2016;25:217-25. [Crossref] [PubMed]
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-21. [Crossref] [PubMed]
- Partelli S, Bartsch DK, Capdevila J, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017;105:255-65. [Crossref] [PubMed]
- Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg 1995;169:36-42; discussion 42-3. [Crossref] [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [Crossref] [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [Crossref] [PubMed]
- Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery 2018;163:218-25. [Crossref] [PubMed]
- Partelli S, Inama M, Rinke A, et al. Long-Term Outcomes of Surgical Management of Pancreatic Neuroendocrine Tumors with Synchronous Liver Metastases. Neuroendocrinology 2015;102:68-76. [Crossref] [PubMed]
- Chawla A, Williams RT, Sich N, et al. Pancreaticoduodenectomy and metastasectomy for metastatic pancreatic neuroendocrine tumors. J Surg Oncol 2018;118:983-90. [Crossref] [PubMed]
- Yuan CH, Wang J, Xiu DR, et al. Meta-analysis of Liver Resection Versus Nonsurgical Treatments for Pancreatic Neuroendocrine Tumors with Liver Metastases. Ann Surg Oncol 2016;23:244-9. [Crossref] [PubMed]
- Lesurtel M, Nagorney DM, Mazzaferro V, et al. When should a liver resection be performed in patients with liver metastases from neuroendocrine tumours? A systematic review with practice recommendations. HPB (Oxford) 2015;17:17-22. [Crossref] [PubMed]
- Aoki T, Kubota K, Kiritani S, et al. Survey of surgical resections for neuroendocrine liver metastases: A project study of the Japan Neuroendocrine Tumor Society (JNETS). J Hepatobiliary Pancreat Sci 2021;28:489-97. [Crossref] [PubMed]
- Zhang XF, Beal EW, Chakedis J, et al. Early Recurrence of Neuroendocrine Liver Metastasis After Curative Hepatectomy: Risk Factors, Prognosis, and Treatment. J Gastrointest Surg 2017;21:1821-30. [Crossref] [PubMed]
- Spolverato G, Bagante F, Aldrighetti L, et al. Management and outcomes of patients with recurrent neuroendocrine liver metastasis after curative surgery: An international multi-institutional analysis. J Surg Oncol 2017;116:298-306. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [Crossref] [PubMed]
- Maxwell JE, Sherman SK, O'Dorisio TM, et al. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery 2016;159:320-33. [Crossref] [PubMed]
- Strosberg J, Kunz PL, Hendifar A, et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)Lu-Dotatate: an analysis of the NETTER-1 study. Eur J Nucl Med Mol Imaging 2020;47:2372-82. [Crossref] [PubMed]
- Kaemmerer D, Twrznik M, Kulkarni HR, et al. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann Surg 2021;274:e45-53. [Crossref] [PubMed]
- Neuroendocrine Tumor Research Foundation. Liver Mets Xavier Keutgen, MD University of Chicago. Youtube. 2019. Available online: https://www.youtube.com/watch?v=KnsQT1ILgk0
- McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery 1990;108:1091-6. [PubMed]
- Scoville SD, Xourafas D, Ejaz AM, et al. Contemporary indications for and outcomes of hepatic resection for neuroendocrine liver metastases. World J Gastrointest Surg 2020;12:159-70. [Crossref] [PubMed]
- Heidenreich BM, Kemp Bohan PM, Flor RJ, et al. Examining Perioperative Risk Associated with Simultaneous Resection of Primary Neuroendocrine Tumors and Synchronous Hepatic Metastases. World J Surg 2021;45:531-42. [Crossref] [PubMed]
- Scott AT, Breheny PJ, Keck KJ, et al. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2019;165:166-75. [Crossref] [PubMed]
- De Jong MC, Farnell MB, Sclabas G, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg 2010;252:142-8. [Crossref] [PubMed]
- Xiang JX, Zhang XF, Beal EW, et al. Hepatic Resection for Non-functional Neuroendocrine Liver Metastasis: Does the Presence of Unresected Primary Tumor or Extrahepatic Metastatic Disease Matter? Ann Surg Oncol 2018;25:3928-35. [Crossref] [PubMed]
- de Mestier L, Lardière-Deguelte S, Brixi H, et al. Updating the surgical management of peritoneal carcinomatosis in patients with neuroendocrine tumors. Neuroendocrinology 2015;101:105-11. [Crossref] [PubMed]
- Kianmanesh R, Ruszniewski P, Rindi G, et al. ENETS consensus guidelines for the management of peritoneal carcinomatosis from neuroendocrine tumors. Neuroendocrinology 2010;91:333-40. [Crossref] [PubMed]
- Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012;95:120-34. [Crossref] [PubMed]
- Zhou B, Zhan C, Ding Y, et al. Role of palliative resection of the primary pancreatic neuroendocrine tumor in patients with unresectable metastatic liver disease: a systematic review and meta-analysis. Onco Targets Ther 2018;11:975-82. [Crossref] [PubMed]
- Bertani E, Fazio N, Radice D, et al. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur J Surg Oncol 2017;43:372-9. [Crossref] [PubMed]
- Bertani E, Falconi M, Grana C, et al. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. Int J Surg 2015;20:58-64. [Crossref] [PubMed]
- Bertani E, Fazio N, Radice D, et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann Surg Oncol 2016;23:981-9. [Crossref] [PubMed]
- Tierney JF, Chivukula SV, Wang X, et al. Resection of primary tumor may prolong survival in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2019;165:644-51. [Crossref] [PubMed]
- Kim J, Zimmerman MA, Hong JC. Liver transplantation in the treatment of unresectable hepatic metastasis from neuroendocrine tumors. J Gastrointest Oncol 2020;11:601-8. [Crossref] [PubMed]
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017;162:525-36. [Crossref] [PubMed]
- Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg 2011;146:953-8. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Coppa J, et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases From Neuroendocrine Tumors. Am J Transplant 2016;16:2892-902. [Crossref] [PubMed]
- Le Treut YP, Grégoire E, Klempnauer J, et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg 2013;257:807-15. [Crossref] [PubMed]


