
Ascites and complications: getting to the root of the trouble
After nearly a decade, the American Association for the Study of Liver Diseases has updated its guidance on the diagnosis, evaluation, and management of ascites and its complications (1).
Ascites is the most common first hepatic decompensating event, thereby denoting the transition to from compensated advanced chronic liver disease (cACLD) to decompensated cirrhosis—(literally) a watershed moment in the natural history of liver disease (2). Notably, first decompensation with ascites is associated with a poorer prognosis, as compared to bleeding, indicating that prevention is key (3). In this regard, Baveno VII (4) introduced the concept of non-invasively diagnosing and treating [with non-selective betablockers (NSBB), preferably carvedilol] the at-risk population—i.e., those with clinically significant portal hypertension (CSPH)—to prevent the occurrence of first hepatic decompensation, in particular ascites (5).
Ascites is a consequence of two key disease-driving mechanisms: portal hypertension and systemic inflammation (2). However, in analogy to ‘What therefore God hath joined together, let not man put asunder’ one may argue that what nature has joined, should not be segregated by the scientific community—i.e., portal hypertension and systemic inflammation are closely interrelated and basically two sides of the same medal, emphasizing the need for integrative concepts.
Seen from a hemodynamic perspective, ascites can be modelled as a function of circulating volume (i.e., right atrial pressure) and portal/hepatic venous pressure gradient (6). While ascites is often accompanied by effective hypovolemia, paradoxically, diuretics remain the therapeutic mainstay as they reduce ascites by further lowering central venous pressure. Notably, diuretics and—to an even higher extent—therapeutic large-volume paracentesis are symptomatic treatments acting downstream of a pathophysiological cascade (Figure 1). Decreasing portal/hepatic venous pressure gradient would act upstream, however, while the potency of NSBB is sufficient for reducing the risk of ascites occurrence in cACLD patients (2), they are not sufficiently potent for preventing the recurrence of ascites. Notably, NSBB may be even be detrimental in patients with the most severe form of (refractory) ascites, in whom they may cause paracentesis-induced circulatory dysfunction and reduce renal perfusion (7,8). In detail, NSBB therapy should be dose-reduced or discontinued in case of persistently low blood pressure (systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg) and/or hepatorenal syndrome-acute kidney injury. Moving from NSBB to transjugular intrahepatic portosystemic shunt (TIPS)—a substantially more potent intervention for ameliorating portal hypertension—its placement has previously been indicated in patients with refractory ascites, as defined by strict diagnostic criteria. In initial studies investigating these patients, placement of uncovered TIPS decreased the need for paracentesis, however, this did not translate into a survival benefit (9). Notably, patients with refractory ascites usually show significant systemic inflammation (10,11) and cirrhotic cardiomyopathy is common. The latter may lead to a paradoxical decrease in cardiac output, which—in the context of systemic inflammation-induced vasodilation—may result in low blood pressure. Besides lowering the portal pressure gradient, TIPS placement shifts volume from the congested splanchnic to the systemic circulation, thereby inducing cardiac stress, which may be particularly problematic in patients with refractory ascites, due to its potential link with cirrhotic cardiomyopathy. Moreover, TIPS may be considered contraindicated in those with a MELD ≥18 points or portopulmonary hypertension (1), arguing for early evaluation of TIPS placement, when hepatic function is still preserved and the prevalence of portopulmonary hypertension is low. These considerations are further supported by a recent small RCT (12) indicating that covered TIPS placement in patients with recurrent ascites (≥3 large volume paracenteses/year; refractory ascites excluded) leads to a profound improvement of 1-year-survival—93% vs. 53%. This translates into a number-needed to treat of 2.5, i.e., a magnitude of effect that is an absolute rarity in modern medicine. Although this aspect has also been addressed by the updated AASLD Guidance (1), TIPS is still listed as a treatment for refractory ascites. Thus, we would like to promote the early use of TIPS in the hierarchy of treatments for ascites, with recurrent rather than refractory ascites being the optimal timepoint for TIPS placement, as it has disease-modifying properties in the former patients.
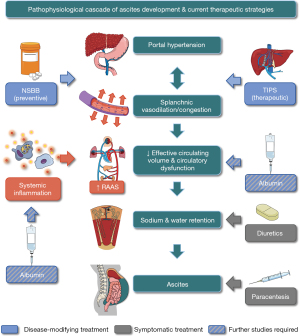
In the ANSWER trial (13), long-term albumin therapy—another emerging disease-modifying treatment—targeted a quite similar patient population (i.e., non-refractory ascites). However, systemic inflammation/circulatory dysfunction (14) rather than portal hypertension is the main therapeutic target of albumin. During a follow-up period of 18 months, survival was 77% with long-term albumin vs. 66% with standard of care. Importantly, the study suffered from lack of blinding, performance bias (long-term albumin patients have been followed more closely), and possibly also Hawthorne effect (closer follow-up in those on long-term albumin may have induced behavioral changes). Notably, most of these concerns may also apply to the previously mentioned TIPS study, nevertheless, the risk reduction seemed to be more profound with TIPS placement. Accordingly, while we are awaiting the results of the PRECIOSA trial (NCT03451292), TIPS rather than long-term albumin is the treatment of choice for recurrent (and refractory) ascites.
Moving to the surgical focus of HBSN, the current version of the AASLD Guidance (1) also provides helpful advice for the management of abdominal hernias, a common and important clinical problem in patients with cirrhosis. In this context, preoperative TIPS placement is mentioned, as effective ascites control is a prerequisite for favorable surgical outcomes. Notably, evidence on preoperative TIPS placement is limited and for other types of surgeries, its role remains unclear (15).
Spontaneous bacterial peritonitis (SBP) is a severe complication of ascites and seen as a consequence of bacterial translocation from the gut, which can be targeted by antibiotic prophylaxis. However, most of the evidence supporting antibiotic prophylaxis for preventing SBP and its sequelae is quite dated, which is particularly worrisome when considering the well-documented changes in microbial epidemiology/susceptibility in cirrhosis. While secondary prophylaxis with norfloxacin is well-established, AASLD (1)—in contrast to EASL (3)—abstains from a firm recommendation for primary prophylaxis in high-risk patients. The emergence of the results of the ASEPTIC trial (NCT04395365), which uses a more pragmatic design than the initial landmark study by Fernández et al. and investigates the efficacy of primary prophylaxis with co-trimoxazole 960 mg q.d. in patients with Child-Turcotte-Pugh stage B/C requiring large-volume paracentesis, will hopefully provide updated data.
Finally, the AASLD Guidance (1) adopted the continuous administration of terlipressin, which is better tolerated, as compared to bolus administration (16). This recommendation seems particularly far-seeing in the light of more recently emerging concerns about increased rates of respiratory failure in terlipressin-treated patients (17,18).
Notably, the AASLD refers to their document as a guidance rather than a guideline, as a sufficient number of randomized-controlled trials for the latter was not available (1). Nevertheless, it seems that hepatologists are getting to the root of the ascites trouble by preventive measures (NSBB therapy) and timely (i.e., already at the stage of recurrent ascites) disease-modifying intervention (TIPS placement), acting ‘upstream’ in the pathophysiological cascade. Targeting systemic inflammation/circulatory dysfunction with long-term albumin may add to the armamentarium, if its effectiveness is confirmed by further trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-625/coif). MM served as a speaker and/or consultant and/or advisory board member for AbbVie, Collective Acumen, Gilead, and Takeda, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. BS received travel support from AbbVie and Gilead. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1014-48. [Crossref] [PubMed]
- Mandorfer M, Simbrunner B. Prevention of First Decompensation in Advanced Chronic Liver Disease. Clin Liver Dis 2021;25:291-310. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959-74. [Crossref] [PubMed]
- Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2019;393:1597-608. [Crossref] [PubMed]
- Levitt DG, Levitt MD. Quantitative modeling of the physiology of ascites in portal hypertension. BMC Gastroenterol 2012;12:26. [Crossref] [PubMed]
- Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis 2017;49:3-10. [Crossref] [PubMed]
- Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol 2020;73:1404-14. [Crossref] [PubMed]
- D'Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology 2005;129:1282-93. [Crossref] [PubMed]
- Turco L, Garcia-Tsao G, Magnani I, et al. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol 2018;68:949-58. [Crossref] [PubMed]
- Costa D, Simbrunner B, Jachs M, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol 2021;74:819-28. [Crossref] [PubMed]
- Bureau C, Thabut D, Oberti F, et al. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology 2017;152:157-63. [Crossref] [PubMed]
- Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417-29. [Crossref] [PubMed]
- Fernández J, Clària J, Amorós A, et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients With Decompensated Cirrhosis. Gastroenterology 2019;157:149-62. [Crossref] [PubMed]
- García-Pagán JC, Saffo S, Mandorfer M, et al. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep 2020;2:100122. [Crossref] [PubMed]
- Simbrunner B, Trauner M, Reiberger T, et al. Recent advances in the understanding and management of hepatorenal syndrome. Fac Rev 2021;10:48. [Crossref] [PubMed]
- Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med 2021;384:818-28. [Crossref] [PubMed]
- Wong F, Pappas SC, Reddy KR, et al. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment Pharmacol Ther 2022;56:1284-93. [Crossref] [PubMed]


