Short and long-term outcomes of laparoscopic compared to open liver resection for colorectal liver metastases
Introduction
Minimally invasive surgery (MIS) is now established as standard of care for a number of surgical procedures in both benign and malignant diseases (1-8). Advanced MIS procedures are being performed by a growing number of surgeons in various practice settings (9). Mainly owing to reduced pain, blood loss, length of hospital stay and complications, as well as faster recovery, laparoscopic offers significant benefits over laparotomy for a variety of abdominal conditions (1-8).
Hepatectomy remains a major and highly challenging surgery, despite the improved morbidity and mortality profiles now achieved in high volume centres. Because of the localization of the liver in the most cephalic portion of the abdomen and the complex and variable intra-hepatic anatomy, laparoscopic liver resection (LLR) is not straightforward. Some particularities of laparoscopic surgery are more disabling in LLR than other gastrointestinal MIS procedures, including loss of 3-dimensional vision, reduced depth of perception, challenging access to some cephalic areas of the liver, and limited range of motion (10). Such technical factors may explain the ongoing concerns regarding the safety, feasibility, and superiority of LLR compared to open liver resection (OLR). As a result, the uptake of LLR has been slower than in other fields (9,11).
Complete resection with hepatectomy is now well established as the standard of care for curative-intent treatment of colorectal liver metastases (CRLM). Remarkable overall survival (OS) results from 30% to 60% at 5 years can be achieved (12,13). The face of liver resection for CRLM has drastically changed over the past decades; medical perioperative care has improved, indication have broadened, techniques have transitioned towards parenchymal sparing resections, and effective perioperative multimodal oncologic therapies have been introduced (14-17). These changes have rendered the uptake of LLR even more challenging in some aspects.
The evidence supporting the use of LLR remains of low quality as currently highlighted by the second LLR Consensus Conference held in Morioka in 2014, even more so when looking specifically at the safety and efficacy of LLR in the treatment of CRLM (18). Therefore, we sought to review the short and long-term outcomes of LLR compared to OLR in the treatment of CRLM.
Methods
Search strategy
In conjunction with an information specialist, we systematically searched Medline (1966–December 2014), Embase (1974–December 2014), the Cochrane Central Register for Controlled Trials, Web Of Knowledge, and the Scopus database (1966–December 2014) to identify potential randomized controlled trials (RCT) and non-randomized studies (NRS), without language or other limitations. Two authors (KAB and JH) selected studies and extracted data independently.
Study selection
Our explicit eligibility criteria included RCT or NRS reporting the effects on short and long-term outcomes of LLR compared to OLR for CRLM. Studies including at least 10 adults (≥18 years old) undergoing liver resection for CRLM were eligible. Studies that included patients not fulfilling our inclusion criteria were excluded if we were not able to distinguish those patients from the larger population. In the event of duplicate publication, we included the most relevant and the most informative study.
Data abstraction and outcome measures
We developed and pilot tested a standardized data extraction form following the recommendations of the Cochrane Effective Practice and Organization of Care Review Group (19). Our primary outcome was recurrence-free survival (RFS) and OS. Secondary outcomes included operating time (minutes), estimated blood loss (mL), 30-day post-operative major morbidity defined as Clavien grade 3 to 5 complications (or as per the authors’ definition if Clavien classification was not used), post-operative mortality (20), grade B and C post-hepatectomy liver failure (International Study Group on Liver Surgery classification) (21), R0 resection, margins (cm), and length of stay (LOS) (days).
We used the grade system to present a summary of findings and rate the overall strength of evidence (22).
Statistical analysis
We presented descriptive statistics as means and standard deviation (SD) for continuous variables, and proportions with 95% confidence interval (CI) for dichotomous variables. When studies presented medians and range, we estimated the mean and SD with the method of Hozo (23). Meta-analysis was conducted using Review Manager (RevMan) Version 5.2.5 (The Cochrane Collaboration, Copenhagen, 2012) for each outcome with data in two or more studies. We pooled the data for each outcome using random effects models. Relative risk (RR) with 95% CI was calculated for dichotomous outcomes. We used the I2 statistic to assess the extent of heterogeneity (24). For all tests and CIs we used a two tailed type I error rate of 5%.
Results
Systematic search
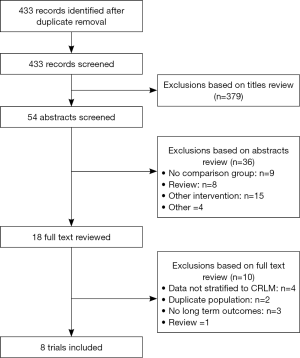
The initial electronic search identified 433 citations, from which eight NRSs enrolling a total of 2,017 patients (580 undergoing LLR) (Figure 1) were selected for inclusion in this review (25-32). Among studies excluded after full text review, two were for duplicate populations (33,34).
Description of included studies
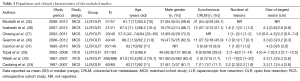
All studies were published in English between 2010 and 2014. They included from 42 to 1,152 patients (Table 1). Six studies used matching techniques to create a comparison cohort of OLR. Criteria for matching for each study of those studies were: (I) number and size of lesions, segmental position, extent of hepatectomy, type of hepatectomy, and time period of resection (32); (II) lesions size (26); (III) tumor stage, number and size of lesions, extent of hepatectomy (undefined) (27); (IV) lesions size (30);(V) propensity score including age, gender, primary tumor localization, number, size, and bilaterality of lesions, presence of extra-hepatic disease, and pre-hepatectomy chemotherapy (25), and (VI) propensity score including age, number and size of lesions, extent of hepatectomy, synchronous colectomy, and clinical risk score (31).
Full table
In studies that did not using a matched analysis, there was a tendency towards more patients with a higher number of lesions (26,28-30) and larger lesions in the OLR group (28,29). The proportion of major liver resections varied between studies, from 5.0% to 62% for LLR, and 5.0% to 62.4% for OLR. In studies that did not match for the extent of hepatectomy, major resections were more common with LLR in one study (26), and with OLR in three studies (25,28-30).
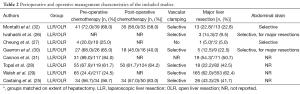
Details of perioperative management are provided in Table 2. Most studies used a totally laparoscopic technique with specimen extraction through a supra-pubic (Pfannenstiel) incision (25-28,30,32).
Full table
Short-term post-operative outcomes
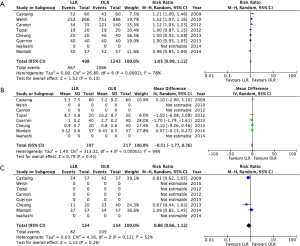
Results of the pooled analysis for short-term post-operative outcomes are presented in Figure 2.
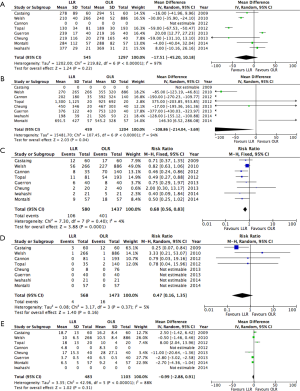
No significant difference was identified between LLR and OLR in terms of blood loss, operative time, or LOS. While none of the matched studies reported a difference in LOS (25-27,30,32), the two unmatched studies outlined fewer days in hospital with LLR (28,29).
LLR was associated with a reduction in the risk of major morbidity (RR: 0.68; 95% CI, 0.56–0.83). Five out of the six matched studies did not report a significant difference in morbidity with LLR (25-27,30,32). The two unmatched studies observed a reduction (28,31). No difference was observed in post-operative mortality (RR: 0.47; 95% CI, 0.16–1.35). Four studies reporting no mortality in either LLR or OLR groups (25,28,29,31).
Oncological outcomes
Pooled risk estimates for oncological outcomes are presented in Figure 3. LLR was not associated with any significant difference in either resection margins or proportion of R0 resections (RR: 1.05; 95% CI, 0.99–1.12). Recurrence did not differ between LLR and OLR (RR: 0.86; 95% CI, 0.66–1.12).
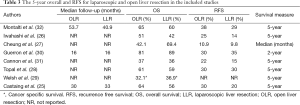
OS and RFS did not differ significantly in any of the included studies. Individual results are presented in Table 3. Five-year OS ranged from 36 to 60% for LLR, and 37–65% for OLR. Five-year RFS varied from 14% to 30% for LLR, and 22% to 38% for OLR. Median follow-up was not reported in most studies. The included studies did not provide enough information to allow for pooling of survival data (number of events and/or hazard ratios).
Full table
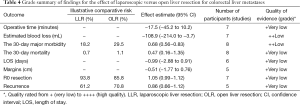
Strength of evidence for each pooled risk estimate is presented in Table 4. According to the grade system, the strength of evidence was low to very low for all considered outcomes.
Full table
Discussion
Due to benefits in terms of operative efficiency as well as post-operative pain, morbidity, and recovery, MIS has become standard of care in various gastrointestinal procedures for both benign and malignant diseases (3,6,35,36). MIS training has been formally incorporated in surgical curriculums, and more advanced procedures are being performed by larger groups of surgeons (9,37,38). Since Reich et al. reported the first LLR in the early 1990’s, its uptake has been rather slow (39). With the advent of new technologies and laparoscopic instruments, the feasibility of LLR has improved and its adoption has recently started to increase (18,40,41). The proportion of all hepatectomies performed laparoscopically however still remains low—around 25% in single centres reports and 14% in nationwide European experiences (42,43). CRLM represent only a small portion of those LLRs. In a recent worldwide review, 50% of LLRs were performed for malignancy. Of those, 35% were CRLM. Indeed, the French experience indicates that only 7.4% of 3,044 hepatectomies performed for CRLM from 2006 to 2014 were done laparoscopically (44).
MIS offers an opportunity to reduce surgical morbidity and enhance post-operative recovery. Thus, increasing the use of LLR is important in improving outcomes for CRLM. Animal studies have revealed that laparoscopic approach results in reduced stress response to surgery as evidenced by changes in interleukin-6, tumor necrosis factor, and adhesion formation, when compared to laparotomy for liver resection (45). The results from this review confirm previous reports of lower morbidity with LLR compared to OLR for CRLM (RR: 0.68). However, this did not translate into shorter LOS in the current analysis. Most studies included in this review were matched, which may explain the difference with prior reports indicating reduced operative time and LOS (46-48). It is important to stress that the results reported here are based on pooled estimates from retrospective studies with small sample sizes, inherently susceptible to bias.
Concerns regarding the benefits of LLR for CRLM have been voiced regarding the ability to identify small lesions and achieve negative margins (49). Older issues pertaining to the trophic effect of the pneumoperitoneum and port site metastases have been dismissed in assessments of colorectal cancers, including large RCT of laparoscopic colectomy (50,51). Those results can be extrapolated to CRLM. Long-term outcomes have not often been compared between LLR and OLR for CRLM. Most studies focused on technical feasibility and short-term outcomes. Therefore, we chose to consider only studies that reported on oncologic outcomes, including survival. Although data could not be pooled, none of the included studies identified inferior survival with LLR. The limited number of studies reporting on long-term outcomes after LR for CRLM, as well as their frequently small sample sizes and single-institution nature, has to be considered when interpreting the results of this review. As previously mentioned, LLR represents a challenging technique. Not many centres have adopted it. Those who have face selection biases in deciding which patients to approach laparoscopically and which ones to operate on with laparotomy. The relatively recent increase in the use of LLR for CRLM also influences the availability of long-term data to report on. Therefore, it is difficult to draw definitive conclusions as to whether LLR can achieve similar oncologic outcomes as OLR.
Being able to provide the patient with similar resection laparoscopically and open is important when looking at both short and long-term outcomes. CRLM present specific challenges for LLR; pre-hepatectomy chemotherapy alters the quality of the liver parenchyma and may render it more prone to bleeding, and parenchyma preserving procedures are paramount (14,52). The latter has been reported to significantly reduce morbidity and mortality of hepatectomy for CRLM, while providing excellent long-term outcomes with the potential for beneficial repeat resection in the face of recurrence (14,52). LLR provides better magnification of operative field than with OLR, which can help in performing a precise resection. However, other technical issues can potentially hamper the ability to save liver parenchyma. The analysis of visual and tactile stimuli necessary to properly assess the complex intra-hepatic anatomy in order to perform precise, safe, and parenchymal-sparing liver resections, is rendered even more challenging by the loss of tactile feedback, lack of 3-dimensional visualization, and difficult hand-eye coordination with laparoscopy. Therefore, concerns still exist regarding the feasibility of LLR for CRLM while meeting current oncologic resection standards. Larger pieces of liver parenchyma may have to be resected laparoscopically to treat the same lesion. This problem was highlighted in the recommendations from the recent Second Consensus on LLR held in Morioka in 2014 (18). Unfortunately, it could not be assessed in this review.
No one can deny the repeatedly reported benefits of laparoscopic surgery over laparotomy for gastrointestinal procedures (1-8,35). However, when looking at LLR for CRLM, one has to carefully consider patient and lesion selection to ensure that the benefits remain higher than the potential downsides of the laparoscopic technique. LLR has not yet reached the level of standard of care for CRLM resection. The appropriateness of the surgical approach has to be tailored to the patient medical condition and the disease pattern in the liver. Liver parenchyma should not be sacrificed for the sake of performing the hepatectomy laparoscopically. Selection for LLR has to be based on the patient’s ability to tolerate a potentially prolonged surgical intervention, and the number, localization, and size of lesions to be resected. Considering the long learning curve for LLR, the expertise of the surgeon also needs to be taken into consideration (11,53). Tools such as the newly described Morioka score can assist surgeons in this selection process by providing an objective appreciation of the complexity of LLR for a given patient. This scoring system is based on the size of lesions, extent of resection required, location within the liver, proximity to major vessels, and degree of fibrosis (54). However, it does not consider parenchymal sparing resections nor does it pertain specifically to CRLM.
Important limitations exist among the studies included in this review, mostly due to their small sample sizes, retrospective designs, and lack of multivariable analyses. However, this review is based on a comprehensive, systematic and highly sensitive literature search that was conducted without restriction for language or the type of publication. Including non-randomized designs allowed for a thorough review of the available literature. Thus, this review offers a systematic and objective assimilation of the available data regarding the long-term outcomes of LLR compared to OLR, as well as insight about the particularities of LLR for CRLM.
Conclusions
Based on limited retrospective evidence, LLR offers reduced morbidity and blood loss when compared to OLR in the surgical treatment of CRLM. Comparable oncologic outcomes can be achieved with LLR and OLR in terms of resection margins, R0 resection, recurrence, and long-term survival results. LLR for CRLM presents specific challenges, mainly pertaining to the feasibility of parenchymal-sparing resection. LLR cannot be considered as standard of care for CRLM at the moment. The decision to proceed with LLR over OLR rests on careful patient and lesion selection to ensure optimal risk-benefits balance. However, LLR represent a paramount tool in the liver surgeon’s armamentarium. Surgeons should be proficient with LLR in order to be able to offer it to properly selected patients and provide them with the benefits of MIS when feasible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Schlachta CM, Mamazza J, Gregoire R, et al. Could laparoscopic colon and rectal surgery become the standard of care? A review and experience with 750 procedures. Can J Surg 2003;46:432-40. [PubMed]
- Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg 1999;23:389-96. [Crossref] [PubMed]
- Glasgow RE, Yee LF, Mulvihill SJ. Laparoscopic splenectomy. The emerging standard. Surg Endosc 1997;11:108-12. [Crossref] [PubMed]
- Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006.CD006231. [PubMed]
- Kuo PC, Johnson LB, Sitzmann JV. Laparoscopic donor nephrectomy with a 23-hour stay: a new standard for transplantation surgery. Ann Surg 2000;231:772-9. [Crossref] [PubMed]
- Lujan J, Valero G, Biondo S, et al. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc 2013;27:295-302. [Crossref] [PubMed]
- Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061-8. [Crossref] [PubMed]
- Hallet J, Labidi S, Bouchard-Fortier A, et al. Oncologic specimen from laparoscopic assisted gastrectomy for gastric adenocarcinoma is comparable to D1-open surgery: the experience of a Canadian centre. Can J Surg 2013;56:249-55. [Crossref] [PubMed]
- Hallet J, Mailloux O, Chhiv M, et al. The integration of minimally invasive surgery in surgical practice in a Canadian setting: results from 2 consecutive province-wide practice surveys of general surgeons over a 5-year period. Can J Surg 2015;58:92-9. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
- Martin RC, Scoggins CR, McMasters KM. Laparoscopic hepatic lobectomy: advantages of a minimally invasive approach. J Am Coll Surg 2010;210:627-34, 634-6.
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [Crossref] [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. [Crossref] [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-17. [Crossref] [PubMed]
- de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg 2008;248:626-37. [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell; 2008.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Castaing D, Vibert E, Ricca L, et al. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg 2009;250:849-55. [Crossref] [PubMed]
- Iwahashi S, Shimada M, Utsunomiya T, et al. Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc 2014;28:80-4. [Crossref] [PubMed]
- Cheung TT, Poon RT, Yuen WK, et al. Outcome of laparoscopic versus open hepatectomy for colorectal liver metastases. ANZ J Surg 2013;83:847-52. [Crossref] [PubMed]
- Topal B, Tiek J, Fieuws S, et al. Minimally invasive liver surgery for metastases from colorectal cancer: oncologic outcome and prognostic factors. Surg Endosc 2012;26:2288-98. [Crossref] [PubMed]
- Welsh FK, Tekkis PP, John TG, et al. Open liver resection for colorectal metastases: better short- and long-term outcomes in patients potentially suitable for laparoscopic liver resection. HPB (Oxford) 2010;12:188-94. [Crossref] [PubMed]
- Guerron AD, Aliyev S, Agcaoglu O, et al. Laparoscopic versus open resection of colorectal liver metastasis. Surg Endosc 2013;27:1138-43. [Crossref] [PubMed]
- Cannon RM, Scoggins CR, Callender GG, et al. Laparoscopic versus open resection of hepatic colorectal metastases. Surgery 2012;152:567-73; discussion 573-4. [Crossref] [PubMed]
- Montalti R, Berardi G, Laurent S, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol 2014;40:536-44. [Crossref] [PubMed]
- Topal H, Tiek J, Aerts R, et al. Outcome of laparoscopic major liver resection for colorectal metastases. Surg Endosc 2012;26:2451-5. [Crossref] [PubMed]
- Doughtie CA, Egger ME, Cannon RM, et al. Laparoscopic hepatectomy is a safe and effective approach for resecting large colorectal liver metastases. Am Surg 2013;79:566-71. [PubMed]
- Sauerland S, Jaschinski T, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2010.CD001546. [PubMed]
- Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004;363:1187-92. [Crossref] [PubMed]
- Palter VN, Grantcharov TP. Development and validation of a comprehensive curriculum to teach an advanced minimally invasive procedure: a randomized controlled trial. Ann Surg 2012;256:25-32. [Crossref] [PubMed]
- Fowler DL, Hogle N. The impact of a full-time director of minimally invasive surgery: clinical practice, education, and research. Surg Endosc 2000;14:444-7. [Crossref] [PubMed]
- Orsenigo E, Baccari P, Bissolotti G, et al. Laparoscopic central pancreatectomy. Am J Surg 2006;191:549-52. [Crossref] [PubMed]
- Gumbs AA, Gayet B, Gagner M. Laparoscopic liver resection: when to use the laparoscopic stapler device. HPB (Oxford) 2008;10:296-303. [Crossref] [PubMed]
- Scatton O, Brustia R, Belli G, et al. What kind of energy devices should be used for laparoscopic liver resection? Recommendations from a systematic review. J Hepatobiliary Pancreat Sci 2015;22:327-34. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Goumard C, Farges O, Laurent A, et al. An update on laparoscopic liver resection: The French Hepato-Bilio-Pancreatic Surgery Association statement. J Visc Surg 2015;152:107-12. [Crossref] [PubMed]
- Allard MA, Cunha AS, Gayet B, et al. Early and Long-term Oncological Outcomes After Laparoscopic Resection for Colorectal Liver Metastases: A Propensity Score-based Analysis. Ann Surg 2015;262:794-802. [Crossref] [PubMed]
- Burpee SE, Kurian M, Murakame Y, et al. The metabolic and immune response to laparoscopic versus open liver resection. Surg Endosc 2002;16:899-904. [Crossref] [PubMed]
- Cai XJ, Yang J, Yu H, et al. Clinical study of laparoscopic versus open hepatectomy for malignant liver tumors. Surg Endosc 2008;22:2350-6. [Crossref] [PubMed]
- Mizuguchi T, Kawamoto M, Meguro M, et al. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today 2011;41:39-47. [Crossref] [PubMed]
- Simillis C, Constantinides VA, Tekkis PP, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms--a meta-analysis. Surgery 2007;141:203-11. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. [Crossref] [PubMed]
- Kuhry E, Schwenk WF, Gaupset R, et al. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008.CD003432. [PubMed]
- Kingham TP, Correa-Gallego C, D'Angelica MI, et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 2015;220:471-9. [Crossref] [PubMed]
- Dagher I, Belli G, Fantini C, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg 2010;211:16-23. [Crossref] [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [Crossref] [PubMed]