STAT3 is a key transcriptional regulator of cancer stem cell marker CD133 in HCC
According to the world cancer report 2014, liver cancer is the second leading cause of cancer related death worldwide. Hepatocellular carcinoma (HCC) is the most common primary liver cancer representing 80–90% of cases and is the final endpoint of chronic liver inflammation caused by viruses, toxins, metabolic liver diseases or autoimmune hepatitis. It is alarming that in the United States, the incidence of HCC has doubled over the past two decades. Due to the late presentation of HCC, a large number of patients are ineligible for potentially curative surgical resection or liver transplantation and have limited chemotherapeutic options. HCC is a heterogeneous complex disease, highly resistant in nature and a significant number of patients experience recurrence after treatment. In recent years, the concept of cancer stem cells (CSCs) has emerged and enabled a better understanding of how tumors successfully evade chemotherapy (1,2).
CSCs are a subpopulation of tumor cells which resemble normal stem cells with respect to their ability to self-renew and differentiate indefinitely. Though CSCs are extremely low in number within a tumor and the specific origin of CSCs remains elusive, growing evidence suggests that CSCs are the tumor initiating cells that play key roles in tumor growth, survival and resistance to chemotherapy and radiation therapy. In recent years, CD133, a CSC marker, has gained significant attention due to its high expression in various human cancers including HCC. CD133 expression was found to be positively correlated with HCC tumorigenicity and chemoresistance (3,4). However, the underlying molecular mechanism of CD133 regulation was not clear. In a recent issue of Hepatology (Hepatology, Vol. 62, No. 4, 2015), Won and colleagues reported a previously unknown mechanism by which the expression of CD133 is increased in the liver tumor microenvironment and promotes the progression of liver carcinogenesis (5). Using human HCC cell lines, they demonstrated that CD133 is encoded by an inducible gene and that its expression at the transcriptional level is directly regulated by interleukin-6 (IL-6) mediated activation of signal transducer and activator of transcription factor 3 (STAT3). Binding of IL-6 to its receptor gp130, causes receptor dimerization and subsequent activation of associated janus kinases (JAKs). Activated JAKs, in turn, phosphorylate the receptor which now serves as the docking site for STAT3 which is also phosphorylated by JAKs. The activated STAT3 translocates into nucleus and directly binds to the promoter of CD133 leading to increased histone acetylation and subsequent transcription of CD133. The authors concluded that IL-6/STAT3-mediated histone modification was therefore key for upregulating CD133 expression under the chronic inflammation conditions leading to HCC. To further validate the finding in vivo, they used a DEN-induced HCC model in TLR4/IL-6 double knockout mice. In absence of IL-6 signaling, STAT3 was not activated and CD133 was not induced.
Hypoxia is a common hallmark of solid tumors, and hypoxia-inducible factor-1 alpha (HIF-1α) is a transcription factor which activates multiple genes in response to hypoxia. In this study, Won and colleagues further demonstrated that the upregulation of CD133 expression was also HIF-1α dependent and that the hypoxic microenvironment contributed to STAT3 mediated upregulation of CD133 expression in cooperation with NFκB-p65. Using chromatin immunoprecipitation (ChIP) assays they observed high enrichment of the active STAT3/NF-κB p65 dimer to the promoter region of HIF-1α. Interestingly, they also observed a synergistic effect of IL-6 and hypoxia on STAT3 activation suggesting that chronic inflammation and hypoxic microenvironment both promote HCC formation by synergistically activating CD133 expression.
Finally, the authors examined the biological role of CD133 in tumorigenesis in vitro and in vivo. Using shRNA mediated gene silencing, they showed that inhibition of CD133 resulted in reduced growth rate of HCC cell lines due to cell cycle arrest and that silencing of CD133 expression also suppressed tumor formation in an HuH-7 xenograft mouse model. Sorafenib, a multi-kinase inhibitor, is the only FDA approved drug currently available for the treatment of HCC, and the authors showed that both sorafenib and nifuroxazide, a STAT3 inhibitor, inhibited tumor growth in vivo by reducing the expression of CD133.
In summary, both IL-6 and hypoxia increase activation of STAT3, a key regulator of CD133 expression and an important mediator in the maintenance of stemness of HCC cells. These observations represent a step forward in understanding the molecular mechanism underlying the enhanced expression of CSC marker CD133 and its role in HCC formation. Interestingly, a previous report had shown that a noncanonical frizzled 2 (FZD2) pathway could also activate STAT3 in HCC, independent of IL-6, and that FZD2-STAT3 signaling induced epithelial to mesenchymal transition (EMT) and was associated with metastasis (6). Thus, it appears that multiple signaling pathways in HCC can activate STAT3 and these events may lead to different phenotypic outcomes (Figure 1). More studies are needed to better understand whether there is any overlap between these signaling mechanisms and how they contribute to EMT, tumor metastasis and stemness.
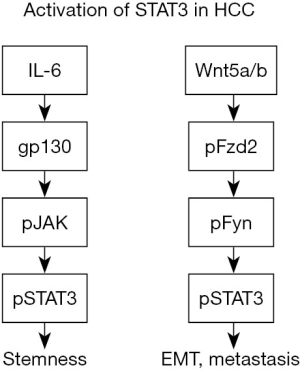
The results of this study suggest that targeting the immune microenvironment by reducing IL-6 mediated inflammation could be another potential therapeutic strategy for HCC, especially for tumors arising in the setting of cirrhosis (7). And in fact a recent study showed that tumor-associated macrophages (TAMs) promote the expansion of CSCs in HCC. Tocilizumab is a recently FDA approved drug that targets IL-6 receptor, and treatment of HCC cells with tocilizumab or STAT3 knockdown reduced the ability of TAMs to not only promote CSC expansion but also growth of xenograft tumors (8). Thus, STAT3 signaling appears to be a promising therapeutic target for HCC treatment.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by the Deputy Editor-in-Chief Haitao Zhao (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 2012;21:283-96. [Crossref] [PubMed]
- Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest 2013;123:1911-8. [Crossref] [PubMed]
- Ma S, Lee TK, Zheng BJ, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008;27:1749-58. [Crossref] [PubMed]
- Yin S, Li J, Hu C, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer 2007;120:1444-50. [Crossref] [PubMed]
- Won C, Kim BH, Yi EH, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology 2015;62:1160-73. [Crossref] [PubMed]
- Gujral TS, Chan M, Peshkin L, et al. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 2014;159:844-56. [Crossref] [PubMed]
- Wiest R, Weigert J, Wanninger J, et al. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine 2011;53:178-83. [Crossref] [PubMed]
- Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014;147:1393-404. [Crossref] [PubMed]


