Losartan may inhibit the progression of liver fibrosis in chronic HCV patients
Introduction
The landscape of treatment for HCV infection has evolved substantially since the introduction of highly effective HCV protease inhibitor therapies in 2011. The pace of change is expected to increase rapidly, as numerous new drugs with different mechanisms of action will likely become available over the next few years (1). However, alternative medical strategies to reduce hepatic fibrosis are under investigation as drugs with antifibrotic effects may be an option in the treatment of patients with chronic HCV who are not eligible or non-responders to anti HCV therapy (2).
In vitro studies have shown that angiotensin II (ANGII) is a mitogenic protein for a number of cell types and between them are the HSCs. In normal human liver, hepatic stellate cells (HSC) do not express ANGII type 1 (AT1) receptors nor do they secrete ANGII. Following chronic liver injury, however, HSC transform into myofibroblast-like cells and express both ANGII type 1 (AT1R) receptors and generate mature ANGTII, which exerts an array of proinflammatory and profibrogenic actions (3,4), like upregulating the transforming growth factor (TGF) β1 expression via ANGII type 1 receptor (AT1R) (4).
The fact that the actions of ANGII are mediated through AT1R is related to therapeutic interventions preventing these pathogenic effects, since AT1R can be completely blocked by losartan, a specific ANGII receptor antagonist (5-8).
For instance, ANGII stimulated mRNA expression of TGF-β and fibronectin can be reversed by saralasin and losartan, nonselective and specific antagonists for AT1R receptors, respectively (9).
Moreover, patients with chronic HCV who displayed decreased fibrosis in response to losartan administration (50 mg/day for 18 months) showed decreased c-Jun N-terminal kinase (JNK) phosphorylation, an enzyme activated in HSC during hepatic fibrogenesis (10). Also, Moreno et al. (11) proved that targeted delivery of losartan to HSCs led to reduction of advanced liver fibrosis; this may provide a novel therapeutic strategy for patients with chronic liver fibrosis.
Therefore, the aim of this study was to investigate the possible antifibrotic effect of losartan on histopathologic level (not by studying serum biomarkers, profibrogenic markers or digital image analyses (in chronic HCV patients with established fibrosis.
Methods
A prospective study on 50 patients with chronic HCV infection and liver fibrosis, (proved by liver biopsy) were enrolled in this study in Tropical Medicine Department; Theodor Bilharz Research Institute from 2012 to 2013. Among these patients, 36 were previous non-responders to a 12-week combined therapy of Peg/Riba and 2 showed lack of compliance to the mentioned treatment. The remaining 12 patients were either not suitable for interferon therapy due to established cirrhosis (10 patients) or they refused to receive treatment (2 patients).
The cirrhotic patients were not evaluated as a separate group as the statistics would be affected by the small sample size.
The patients were divided randomly (computer-generated) into 2 groups. The 1st group (n=25) was given losartan 50 mg as a single daily dose for 1 year (losartan group) and the 2nd group (n=25) was given silymarin 140 mg t.i.d. (silymarin group). Placebo was not easily available, so, silymarin was used as it has conflicting reports on fibrosis. Written informed consent was obtained from all patients, and the local ethics committee approved the study protocol, based on the 1975 Declaration of Helsinki.
Inclusion criteria: (I) adults aged up to 60 years old; (II) having evident fibrosis on liver biopsy (Ishak score >F1) with compensated liver disease (chronic active hepatitis or Child-A cirrhosis); (III) Patients who did not respond or receive complete course (48 weeks) of combined therapy (Peg/Riba), whether patients with history of unsuccessful antiviral therapy (stopped at least 1 year before enrolment in the study, including non-responders and who discontinued Peg/Riba due to intolerable side effects) or patients who refused (Peg/Riba) therapy.
Patients were recruited from the patients excluded from or did not complete our Egyptian national program for treatment of HCV.
Exclusion criteria: (I) other causes of chronic liver disease rather than HCV; (II) decompensated cirrhosis, evident hepatic focal lesions and/or variceal bleeding; (III) patients with renal impairment (serum creatinine above 3 mg/dL); (IV) candidates (and willing to receive) for interferon therapy; (V) both active drinkers and abstainers.
Patients were subjected to: (I) thorough history and medical examination; (II) liver and kidney function tests, CBC, viral markers for HBV and HCV (HBsAg and HCVAb) and quantitative PCR for HCV; (III) abdominal ultrasound; (IV) liver biopsy at baseline and end of study.
All the patients were outpatients and biochemical tests were done in the outpatient clinic. Clinical and biochemical (liver profile and kidney function tests, CBC, HCV PCR) and abdominal ultrasound follow ups and blood pressure were assessed during the first week and monthly after losartan administration (excluding HCV PCR done at end of study). Liver biopsy was repeated after 1 year of treatment (end of study). During losartan administration, patients did not receive any other medications.
Liver biopsy
The sampling variability of liver fibrosis has been thoroughly investigated. The liver core biopsy specimen represents only a very limited part of the whole liver and fibrosis is heterogeneously distributed. In order to avoid these caveats and limit the risk for false evaluation, we considered that a biopsy specimen of at least 1.5 cm length and 1.4 mm thickness is sufficient for accurate histological evaluation (12,13).
The procedure was done percutaneously, ultrasound guided and on outpatient basis. Liver specimens, previously fixed in formalin, were stained with hematoxylin and eosin and Masson trichrome, and examined by two independent liver pathologists, blinded to the clinical data of the patients. The reciprocal consistence between the two pathologists was satisfactory. Histological activity index and fibrosis stage were assessed by means of Ishak’s score (14). Paired biopsy specimens taken at baseline and at the end of the study were compared.
Statistical analysis
Qualitative data of the 2 groups were presented by number and percent. They were compared by Chi-square or Fischer’s exact test, when appropriate. Quantitative data were expressed by mean and standard deviation (SD). They were compared by unpaired t-student test. Before and after treatment, data in each group were compared by paired t-test. In all tests, P was considered significant if less than 0.05.
Results
The study included 50 patients divided randomly into 2 equal groups, losartan and silymarin groups, however, only 39 patients completed the study (1 year duration), of which 21 were taking losartan and 18 were taking silymarin.
Among the 39 patients who completed the study, only 27 underwent the second (end of trial) liver biopsy, of which 16 were taking losartan and 11 were taking silymarin. The causes of these drop-outs were patients’ refusal to the second liver biopsy at the end of the study (n=12 patients) or earlier refusal of compliance to treatment and follow up (n=11).
The losartan group included 12 (75%) males and 4 (25%) females with a mean age of 44.31±7.29 years, while the silymarin group included 9 (81.8%) males and 2 (18.2%) females with a mean age of 40.55±7.53 years.
There were no significant differences in the arterial blood pressure and biochemical parameters, including liver and kidney functions, blood counts, prothrombin concentration and HCV PCR before starting treatment both in the losartan group and silymarin group (Table 1). Also, there were no significant differences in fibrosis stage and inflammation grade before starting treatment in both groups.
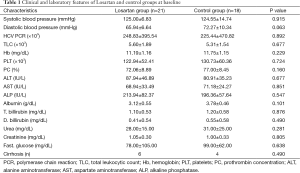
Full table
In the second liver biopsy, after 1 year, the decrease in fibrosis stage was significantly different between the losartan group and silymarin group [a decrease of 1.88±0.96 (50.9%) vs. 0.45±0.93 (11.7%), respectively; P<0.01]. In patients treated with losartan, a decrease in fibrosis stage was observed in 14/16 (87.5%) losartan group vs. 2/11 (18.2%) silymarin group (P<0.01). There were no significant differences observed in grades of inflammation in both groups (Table 2).
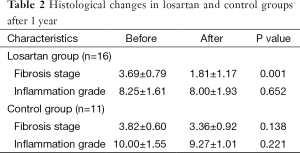
Full table
A significant increase in albumin level of 0.23±0.31 (7%) vs. a decrease of −0.07±0.21 (−1.8%) (P<0.01) and an increase of prothrombin concentration of 5.81±4.69 (8%) vs. a decrease of −0.36±2.25 (−0.46%) (P<0.01) were found at the end of the study in the losartan group when compared to silymarin group respectively. No correlation was found between fibrosis regression and improvement of albumin/prothrombin levels in the losartan group (P>0.05) (Tables 3,4).
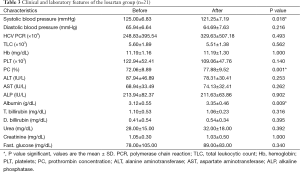
Full table
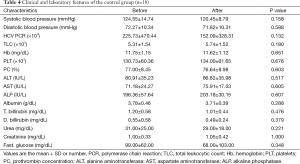
Full table
Moreover, there was a significant decrease in systolic arterial blood pressure at the end of the study in the losartan group, but not in silymarin group (P=0.018) (Tables 3,4).
However, there were no significant changes in the rest of the biochemical parameters, including liver and kidney profiles, blood counts and HCV PCR both before and at the end of the study in both groups (Tables 3,4). No serious side effects were noted during the study.
Discussion
It is well known that, at present, the most effective therapy for treating hepatic fibrosis is the removal of the causative agent. In chronic HCV, this goal may be achieved with antiviral therapy (whether standard or DAAs) in a significant proportion of patients. Nevertheless, non-responders or cirrhotics may obtain a benefit from the emerging antifibrotic therapies (2).
When considering antifibrotic therapy, it is important to recognize that fibrosis is a dynamic process. Although the fibrotic lesion was once believed to be static, abundant evidence indicates otherwise. A prominent feature of liver fibrosis is extracellular matrix turnover, including not only synthesis but also degradation (15). Thus, the fibrogenic process is dynamic, and even advanced fibrosis is reversible.
Many specific antifibrotic treatments have been tested, but none have succeeded. Nonetheless, because of the importance of fibrosis, the field of antifibrotic compounds is rapidly growing (16).
The ANGII system represents an extremely attractive antifibrotic target. Abundant experimental evidence indicates overproduction of ANGII in the injured liver, and a role of ANGII in stimulation of HSC activation and fibrogenesis (17).
Since renin-angiotensin system blockers are promising drugs in reducing the accumulation of scar tissue in experimental models of chronic liver injury and in pilot small scale studies, we performed our study aiming to examine the antifibrotic effect of losartan administration in chronic HCV non-responders or those with contraindications to interferon plus ribavirin regimen.
In our study, histopathological scores showed that losartan had an inhibitory effect on the progression of hepatic fibrosis stage and even led to regression of fibrosis in 87.5% of patients. Also, Sookoian et al. (2) performed a study on 14 chronic HCV patients giving them losartan 50 mg/d for 6 months. The changes in the fibrosis stage were significantly different between the losartan group (decrease of 0.5±1.3) and control group (increase of 0.89±1.27; P<0.03). In the treated patients, a decrease in fibrosis stage was observed in 7/14 patients vs. 1/9 controls (P<0.04). A decrease in sub-endothelial fibrosis was observed in the losartan group. Subendothelial fibrosis was evaluated by digital image analyses. As in a previous study of their group using this method they showed a high correlation between image analysis and fibrosis stage. No differences were found in histological activity index after losartan administration.
Similar results were found by Colmenero et al. (18) who studied 14 patients with HCV and liver fibrosis who received losartan (50 mg/day) for 18 months. In their study, overall collagen content and fibrosis stage remained stable in the whole series, yet the fibrosis stage decreased in 7 patients (50%). Inflammatory activity improved in 7 patients (50%). Moreover, losartan treatment was associated with a significant decrease in the expression of several profibrogenic and NOX (NADPH oxidase) genes including procollagen α1(I) and α1(IV), urokinase-type plasminogen activator, metalloproteinase type 2, NOX activator 1 (NOXA-1) and organizer 1 (NOXO-1), and Rac-1.
Similarly, Kluwe et al. (10) detected a significant reduction of fibrosis in patients who responded to losartan with significant decrease in p-JNK expression (enzyme playing an important role in hepatic fibroblasts and HSC) whereas p-JNK expression remained unchanged in patients who did not respond to Losartan.
Also, Terui et al. (19), in his study that included 30 patients divided into two groups, the degree of fibrosis was expressed as the percentage of the total area measured using a tablet measuring device for micromeasurement. They found that the fibrosis score was not significantly different before and after the study in the losartan group. However, in the losartan group, fibrosis area showed a significant decrease (P<0.001).
In our study, liver and renal biochemical tests and blood cells counts showed no change in either group, except for the prothrombin and the albumin levels in losartan arm, who demonstrated a significant improvement in comparison with the silymarin group. The improvement of albumin and prothrombin levels could be due to decrease of fibrosis leading to better blood supply to hepatocytes on histologic level, this could lead to better function of hepatocytes.
Also, Sookoian et al. (2) found improvement of albumin level, but prothrombin concentration changes were not significant in their study. Additionally Sookoian et al. (2) found significant increase in platelets number in losartan group compared to silymarin group but this was not found in our study. While Colmenero et al. (18) found no effects on serum liver tests.
Also, El-Sisi et al. (20) studied the fibrosis in three groups of chronic HCV, all three groups received silymarin, the one group received telmisartan and the second received losartan. They studied the serum biochemical markers like ALT, AST, GGT, α2 macroglobulin, TGF-α and Urinary hydroxyproline before and after treatment. The losartan group showed non-significant changes while telmisartan treated patients showed a significant increase in platelets and leukocytic counts, significant reductions in the levels of GGT, TGF-α, α2-macroglubulin and urinary hydroxyproline (P<0.05) while silymarin group showed significant increases in TGF-α, α2-macroglobulin and urinary hydroxyproline.
No correlation was found, in our study, between fibrosis regression and improvement of albumin/prothrombin levels in the losartan group, this could be due to the small sample size and needs a larger number to confirm or antagonize this result.
In our study, there was a significant decrease in systolic blood pressure at the end of the study in the losartan group, but not in silymarin group. On the contrary, in El-Sisi et al.’s study (20), a significant decrease in systolic and diastolic blood pressure compared to baseline level was found in telmisartan treated patients but not in losartan or silymarin groups.
Serum HCV-RNA levels showed no change by the end of our study, in confirmation, Sookoian et al. (2) and Colmenero et al. (18) indicated that losartan antifibrotic effect was not related to alleviation of the cause. That is why Czaja (21) reported that losartan had shown safety and efficacy in many clinical trials, and these experiences have strengthened its preference as antifibrotic agent in different causes of liver cirrhosis.
Patients with cirrhosis were not excluded, in our study, aiming at studying the effect of losartan on the advanced stage of fibrosis i.e., cirrhosis; they were also included as some experts may have criticized the exclusion of the most advanced fibrosis stage in our study.
In conclusion, Losartan had an inhibitory effect on progression and even led to regression of the hepatic fibrosis stage but had no effect on grade of inflammation. This was associated with evidence of improvement in synthetic liver functions, namely albumin and prothrombin synthesis.
Losartan action in reversing hepatic fibrosis could lead to prevention of further complications like bleeding varices, ascites and encephalopathy. Also, may lead to decreasing the need for liver transplantations and all of its complications.
The explanation of the improvement of the synthetic capability of the liver in cirrhotics is an ambitious one and demands cautious translation in the clinical setting; nevertheless, the reversal of fibrosis under treatment has been demonstrated for various drugs (more recently antivirals).
Losartan had no effect on HCV-RNA levels, denoting that it has a direct antifibrotic effect and does not work through the etiology of liver fibrosis.
A larger number studies is required to confirm its antifibrotic action and, if proved, losartan may provide an effective new approach to the treatment of non-responders to antiviral therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Written informed consent was obtained from all patients, and the local ethics committee approved the study protocol, based on the 1975 Declaration of Helsinki.
References
- HCV guidance: recommendations for testing, managing, and treating hepatitis C. Available online: http://www.hcvguidelines.org, September 2014.
- Sookoian S, Fernández MA, Castaño G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol 2005;11:7560-3. [Crossref] [PubMed]
- Wei HS, Lu HM, Li DG, et al. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: effects of RAS inhibitors on hepatic fibrosis induced by CCl(4). World J Gastroenterol 2000;6:824-8. [Crossref] [PubMed]
- Bataller R, Sancho-Bru P, Ginès P, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 2003;125:117-25. [Crossref] [PubMed]
- Paizis G, Gilbert RE, Cooper ME, et al. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol 2001;35:376-85. [Crossref] [PubMed]
- Yoshiji H, Kuriyama S, Yoshii J, et al. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001;34:745-50. [Crossref] [PubMed]
- Croquet V, Moal F, Veal N, et al. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol 2002;37:773-80. [Crossref] [PubMed]
- Ramalho LN, Ramalho FS, Zucoloto S, et al. Effect of losartan, an angiotensin II antagonist, on secondary biliary cirrhosis. Hepatogastroenterology 2002;49:1499-502. [PubMed]
- Leung PS, Suen PM, Ip SP, et al. Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept 2003;116:61-9. [Crossref] [PubMed]
- Kluwe J, Pradere JP, Gwak GY, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 2010;138:347-59. [Crossref] [PubMed]
- Moreno M, Gonzalo T, Kok RJ, et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology 2010;51:942-52. [PubMed]
- Colloredo G, Guido M, Sonzogni A, et al. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 2003;39:239-44. [Crossref] [PubMed]
- Saleh HA, Abu-Rashed AH. Liver biopsy remains the gold standard for evaluation of chronic hepatitis and fibrosis. J Gastrointestin Liver Dis 2007;16:425-6. [PubMed]
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696-9. [Crossref] [PubMed]
- Arthur MJ, Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2000;279:G245-9. [PubMed]
- Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis 2008;12:939-62. xi. [Crossref] [PubMed]
- Bataller R, Sancho-Bru P, Ginès P, et al. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal 2005;7:1346-55. [Crossref] [PubMed]
- Colmenero J, Bataller R, Sancho-Bru P, et al. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 2009;297:G726-34. [Crossref] [PubMed]
- Terui Y, Saito T, Watanabe H, et al. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology 2002;36:1022. [Crossref] [PubMed]
- El-Sisi AE, Elfert AA, El-Sayad M, et al. Anti-fibrotic effect of telmisartan in silymarin treated HCV Egyptian patients. Int J Pharm Pharm Sci 2011;3:1706-14.
- Czaja AJ. Review article: The prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther 2014;39:385-406. [Crossref] [PubMed]


