Hypophosphatemia and recovery of post-hepatectomy liver insufficiency
Introduction
Recent major advancements in the technique and intra-operative management have significantly improved outcomes of hepatic surgery (1). However, post-operative liver failure remains a significant complication, responsible for 60% to 100% of deaths after liver resection (1-4). Considering the rising prevalence of parenchymal liver disease and the increased use of potentially hepatotoxic neo-adjuvant chemotherapy, more patients are and will be at risk of post-hepatectomy liver failure (PHLF) (5,6).
While initial liver insufficiency (ILI), whereby serum bilirubin and international normalized ratio (INR) rise in the first 24 to 72 hours following hepatectomy, is frequent, it is most often transient (7) and not predictive of outcomes. Early and reliable identification of patients who will progress towards formal PHLF is an ongoing challenge. Scoring systems have been suggested to standardize PHLF definition, and predict post-hepatectomy morbidity and mortality. Unfortunately, they cannot be used before the fifth post-operative day (8), thus precluding our ability to distinguish early, which patients will recover from ILI and which ones will suffer from PHLF.
Phosphate plays a key role in the formation of high-energy bonds and is essential to multiple metabolic processes. Severe hypophosphatemia (HP) has been associated with a variety of high energy catabolic processes, including re-feeding (9,10) and trauma (11). The frequent occurrence of HP after liver resection has been reported in case reports and small case series (12-14). The meaning of HP in association with liver failure or morbidity remains controversial. While some small observational studies reported an association with increased post-operative morbidity (13,14), others observed no difference (15,16). However, as the liver contains 0.3% of phosphorus by weight (17), liver regeneration is also associated with considerable movement of phosphate into the hepatocytes (18,19). Thus it is also possible that HP could be a surrogate marker for early liver regeneration post hepatectomy.
We sought to examine the association between post-hepatectomy HP and liver insufficiency’s course. We hypothesized that between post-hepatectomy HP was associated with higher rates of ILI, and higher rates of recovery from ILI.
Methods
We conducted a retrospective cohort study approved by the Sunnybrook Health Sciences Centre Research Ethics Board [324-2013].
Selection of participants
Patients undergoing liver resection from January 2009 to June 2012 at a single academic institution specialized in Hepato-Pancreato-Biliary (HPB) surgery (Sunnybrook Health Sciences Centre—The Odette Cancer Centre) were identified using the institutional Liver Database. We included all adult patients (≥18 years old), undergoing liver resection regardless of the indication or extent of resection, with at least one serum phosphate measure in the first 72 hours following surgery. We excluded patients for which no post-operative serum phosphate level was available during this time period.
Based on previous studies reporting on the frequency of moderate to severe post-hepatectomy HP (13,20), we defined HP serum phosphate equal or below 0.65 mmol/L. It is also clinically and biologically sensible that a drop in serum phosphate due to liver regeneration would be larger than that of mild HP (≤0.81 mmol/L).
Outcomes and data collection
Primary outcomes were the occurrence of ILI within the first five post-operative days, defined as serum bilirubin above 50 µmol/L and INR above 1.7 (Prothrombin Time >50%) (8), and recovery from ILI, defined as resolution of those parameters by the day of discharge from hospital. Secondary outcomes were 30-day post-operative major morbidity (Calvien-Dindo grade 3 or 4 complications) (21), mortality, and re-admission.
Assessors not involved in the treatment process collected demographics, clinical, and operative data using a standardized form. Data collection included baseline demographics, diagnostic, operative, and post-operative course details. Nadir serum phosphate (mmol/L) was captured within 72 hours following surgery. Peak bilirubin (µmol/L), and peak INR values were recorded within five days of surgery, and at discharge (on the day of or closest value to the day of discharge). Background liver disease was defined as either fibrosis or cirrhosis reported on pathology, and major hepatic resection as more than three liver segments resected.
All liver resections at our institution are performed aiming for low central venous pressure (22). Patients are monitored for at least 24 hours in a surgical intensive care unit after surgery. Oral diet is resumed as soon as possible, usually on post-operative day 1. Serum phosphate, bilirubin and INR are routinely measured daily after surgery. Standardized order sets for intravenous potassium phosphate or sodium phosphate are routinely used to replete serum phosphate, based on daily measurements.
Statistical analysis
Analysis was performed using SPSS 21.0 (IBM Corp., Amonk, NY, USA). Categorical data were reported as absolute number (n) and proportion (%), and continuous data as median with interquartile range (IQR) or mean with standard deviation (SD). Comparison analysis was conducted between HP and normophosphatemia (NP) groups using the Student t test, Fisher exact test, Mann-Whitney U test or Pearson Chi square test, as appropriate. Results were considered significant at P<0.05.
Results
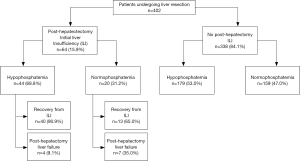
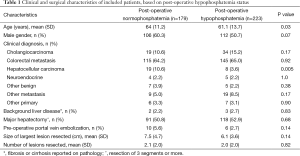
Among 402 patients undergoing liver resection and having post-operative serum phosphate levels available, 223 (55.5%) experienced post-operative HP (Figure 1). No patient had severe HP (serum phosphate ≤0.32 mmol/L). Patients with HP were younger than those with NP and less likely to undergo resection for hepatocellular carcinoma (HCC) (Table 1). There were no other differences in baseline characteristics of patients with and without HP.
Full table
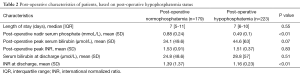
The postoperative course, including liver function characteristics, is detailed in Table 2 and Figure 2. No difference was observed in 30-day post-operative major morbidity, 30-day mortality, 30-day re-admission, and length of stay between HP and NP groups.
Full table
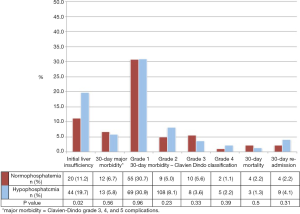
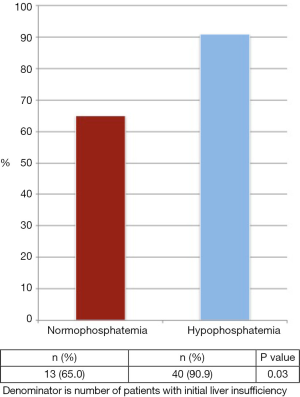
ILI occurred in 15.9% of all patients in the cohort. Patients with ILI were significantly more likely to experience HP compared to those without ILI (68.8% vs. 53.0%, P=0.02). Among all patients with ILI, 82.8% recovered. More patients with HP recovered from ILI compared to those with NP (Figure 3, 90.9% vs. 65.0%, P=0.03).
Discussion
This is one of the largest studies examining the association between post-hepatectomy HP and course of liver insufficiency. We observed no association between post-hepatectomy HP and post-operative morbidity, mortality, re-admission or length of stay. In patients experiencing ILI, recovery of liver function was significantly higher in the presence of HP (90.9% vs. 65.0%; P=0.03).
HP has been reported after various types of surgery, occurring in all patients in one series of aortic bypass (n=10), and 34.3% of 566 patients in two retrospective reviews (23,24) of open-heart surgery,. However, after liver resection, more profound and frequent HP has been observed. In two retrospective cohort studies including respectively 35 and 44 patients undergoing major hepatectomies, 67% and 61.3% rates of HP were identified (13,14). In the living liver donor population, retrospective reports showed HP to occur in 65.9% to 70% of patients after right hepatectomy (15,25,26). We observed a slightly lower proportion of HP patients (55.5%), which can be related to larger sample size, routine post-operative repletion of phosphate, and/or inclusion of all extent of hepatectomies.
The implications of HP following liver resection remain controversial. Some authors have reported increased morbidity in patients with HP, including prolonged mechanical ventilation time and longer length of stay (13,14,25,26). They advocated for early and aggressive repletion of serum phosphate after hepatectomy to prevent surgical complications (13,14,25,26). With a sample size ten times larger than these previous studies, our study did not reveal a difference in post-operative morbidity between HP and NP (5.8% vs. 6.7%; P=0.56). While we acknowledge that inclusion of minor liver resections (≤3 segments) may have lowered the occurrence of post-operative morbidity in the cohort, the large sample size and contemporary cohort provide an accurate estimate. Our works is indeed consistent with a retrospective cohort study supports our observations—among 88 living liver donors, degrees of HP were not associated with a difference in post-operative morbidity (15). Furthermore, a contemporary analysis of 719 major liver resections also reported increased post-operative morbidity and mortality associated with the lack of HP rather than its presence (27).
Our findings suggest that, rather than bearing an increased risk for surgical complications, HP is associated with higher rates of recovery from ILI, and could represent a prognostic indicator of ILI recovery. Data about acute and fulminant liver failure of other causes corroborate our findings. In a review of 38 cases of fulminant hepatic failure referred for liver transplant, mean nadir serum phosphate was lower in those who recovered as compared to those who required transplant or died from liver failure (1.18 vs. 1.79 mg/dL; P=0.02) (16). Lack of HP was identified as predictor of mortality, being even equivalent to King’s College Criteria to prognosticate outcomes of acute liver failure. Better 1-week recovery rate in patients with lower serum phosphate levels (74% if <2.5 mg/dL vs. 45% if 2.5–5.0 mg/dL vs. 0% if >5.0 mg/dL; P=0.0001) was also revealed in an additional retrospective analysis of 112 acute liver failure patients (28). Finally, the only other large contemporary analysis of post-hepatectomy HP indicated that patients presenting an elevated phosphate on post-operative day 2 were at higher risk of liver insufficiency (27).
Many hypotheses have been proposed to explain post-hepatectomy HP, but the exact underlying mechanism is yet to be determined. Phosphate homeostasis is complex, involving kidneys, gastrointestinal absorption, skeletal muscle, as well as numerous endogenous cytokines and humoral substances (29). Beyond liver resection, other common factors of post-operative management have been implicated in HP after surgery, including re-feeding syndrome, parenteral nutrition with low phosphate content, and use of glucose containing intravenous solutions (30-32). These factors were unlikely to be implicated in HP observed in this study. This population included elective, stable, well-nourished patients who routinely resumed diet the day after surgery. In addition, there was no routine use of parenteral nutrition, and none before post-operative day 7.
Liver tissue contains 0.3% of phosphate by weight (17); therefore increased flux of serum phosphate into hepatocytes appears needed for high energy consuming liver regeneration process. Since it takes up to seven days before phosphate starts mobilizing from bones, such a high phosphate consuming process can account for large decreases in serum phosphate early after surgery (30). This hypothesis is supported by canine models subjected to sub-total hepatectomy that led to rapid uptake of radiolabeled phosphate and increased mitotic counts in the regenerating residual liver, resulting in HP (18,19). Fluid shifts and high metabolic demand, characteristics of post-surgical state, may also exacerbate development of HP (33,34). Attributing post-hepatectomy HP to liver regeneration has been challenged by small studies identifying urinary waste with increased fractional urinary phosphate excretion paralleling the serum phosphate drop among 9 to 20 patients (35,36). The mechanism triggering hyperphosphaturia remains unclear, with processes such as action of phosphatins, or release of proteolytic enzymes by the damaged liver to activate phosphaturic hormones being proposed but not investigated yet (30).
Studies looking at the evolution of liver function following hepatectomy have highlighted common ILI that most often recovers, but that can also progress towards formal PHLF associated with high morbidity and mortality (7). Unfortunately, no good indicator of which patients will recover and which will progress to formal or permanent liver failure is currently available. PHLF consensual definitions rely on values on post-operative day 5 (8,37). Other systems have attempted to score the severity of the liver dysfunction but were not designed to predict its occurrence or outcomes (4,8,37). Even though no treatment is available to prevent or treat post-hepatectomy liver failure, we believe that a clinical prognostic factor to identify early on patients at low risk of progressing towards PHLF could ease safe decision-making and faster tracking through the post-operative course.
The main strength of this study is the analysis of a large sample representative of daily HPB practice. We acknowledge the potential information bias associated with data collection through chart review as well as confounders that could affect the results. As previously mentioned, we chose to include both minor and major liver resections, according to current consensus definitions. This was decided on for a more pragmatic appreciation of the daily liver surgery practice. This avoided excluding patients classified at minor liver resections that could be at risk for ILI. Indeed, with the increased use of parenchymal sparing techniques, multiple wedge resections for parenchymal sparing surgery can potentially involve a fewer number of segments, while representing a more extensive parenchymal transection and liver trauma. Thus, traditional classification into minor and major resection may not always offer an accurate differentiation of extent of resection. Indeed, no difference in major liver resections was observed between NP and HP patients. Results of this analysis also depend largely on the definition of HP. We chose to define HP as serum phosphate equal or below 0.65 mmol/L based on previous studies reporting on the frequency of moderate to severe post-hepatectomy HP, and because its biological and clinical significance (13,20). This work represents one of the largest series addressing the association of serum phosphate and post-hepatectomy liver insufficiency, providing evidence beyond observations on small number of patients that are more difficult to generalize. While another recent large cohort has also examined this issue, our work distinguishes itself by focusing on the specific population presenting ILI, a dilemma regularly face by liver surgeons (27). Not only did it examine the impact of HP on short-term outcomes, but it also investigated its potential clinical usefulness to determine the evolution of patients ILI, for whom no tools are currently available.
Conclusions
In this study, post-hepatectomy HP was not associated with worse post-operative outcomes. Among patients with ILI, patients with HP recovered more often from ILI than those with NP, potentially indicating efficient liver regeneration. Drop in serum phosphate could be a promising early prognostic factor for recovery of liver ILI. Further studies are warranted to explore the association of the trend of serum phosphate drop and liver function, as well as its usefulness as a prognostic factor of recovery from ILI.
Acknowledgements
The authors would like to thank Ms. Jessica Truong for her help in the data collection process, and highlight the precious administrative support of Mrs. Vasu Patel in completing this study.
Footnote
Conflicts of Interest: Part of this work was presented as oral presentation at the 2014 Americas Hepato-Pancreato-Biliary Association (AHPBA) Annual Meeting in Miami, FL, and as poster presentation at the 2014 International Hepato-Pancreato-Biliary World Congress in Seoul, Korea. This work is not published or submitted in any other journal.
Ethical Statement: We conducted a retrospective cohort study approved by the Sunnybrook Health Sciences Centre Research Ethics Board [324-2013].
References
- Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188-200. [Crossref] [PubMed]
- Helling TS. Liver failure following partial hepatectomy. HPB (Oxford) 2006;8:165-74. [Crossref] [PubMed]
- McCall J, Koea J, Gunn K, et al. Liver resections in Auckland 1998-2001: mortality, morbidity and blood product use. N Z Med J 2001;114:516-9. [PubMed]
- Schindl MJ, Redhead DN, Fearon KC, et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289-96. [Crossref] [PubMed]
- Bosetti C, Levi F, Lucchini F, et al. Worldwide mortality from cirrhosis: an update to 2002. J Hepatol 2007;46:827-39. [Crossref] [PubMed]
- Chun YS, Laurent A, Maru D, et al. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol 2009;10:278-86. [Crossref] [PubMed]
- Lock JF, Malinowski M, Seehofer D, et al. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg 2012;397:1297-304. [Crossref] [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 2005;242:824-8, discussion 828-9. [Crossref] [PubMed]
- Brooks MJ, Melnik G. The refeeding syndrome: an approach to understanding its complications and preventing its occurrence. Pharmacotherapy 1995;15:713-26. [PubMed]
- Miller SJ. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Nutr Clin Pract 2008;23:166-71. [Crossref] [PubMed]
- Lovén L, Jansson I, Larsson L, et al. Posttraumatic hypophosphataemia and urinary phosphate excretion with and without phosphate supplementation. An experimental study in pigs. Acta Chir Scand 1983;149:233-8. [PubMed]
- Keushkerian S, Wade T. Hypophosphatemia after major hepatic resection. Curr Surg 1984;41:12-4. [PubMed]
- George R, Shiu MH. Hypophosphatemia after major hepatic resection. Surgery 1992;111:281-6. [PubMed]
- Buell JF, Berger AC, Plotkin JS, et al. The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg 1998;133:757-61. [Crossref] [PubMed]
- Lee HW, Suh KS, Kim J, et al. Hypophosphatemia after live donor right hepatectomy. Surgery 2008;144:448-53. [Crossref] [PubMed]
- Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transpl 2003;9:248-53. [Crossref] [PubMed]
- Woodard HQ, White DR. The composition of body tissues. Br J Radiol 1986;59:1209-18. [Crossref] [PubMed]
- Islami AH, Pack GT, Schwartz MK, et al. Regenerative hyperplasia of the liver following major hepatectomy; chemical analysis of the regenerated liver and comparative nuclear counts. Ann Surg 1959;150:85-9. [Crossref] [PubMed]
- Fisher B, Szuch P, Fisher ER. Evaluation of a humoral factor in liver regeneration utilizing liver transplants. Cancer Res 1971;31:322-31. [PubMed]
- Burak KW, Rosen CB, Fidler JL, et al. Hypophosphatemia after right hepatectomy for living donor liver transplantation. Can J Gastroenterol 2004;18:729-33. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bui LL, Smith AJ, Bercovici M, et al. Minimising blood loss and transfusion requirements in hepatic resection. HPB (Oxford) 2002;4:5-10. [Crossref] [PubMed]
- Andersen PT, Nielsen LK, Møller-Petersen J, et al. Severe hypophosphatemia following elective abdominal aortic bypass grafting. Acta Chir Scand 1987;153:641-6. [PubMed]
- Cohen J, Kogan A, Sahar G, et al. Hypophosphatemia following open heart surgery: incidence and consequences. Eur J Cardiothorac Surg 2004;26:306-10. [Crossref] [PubMed]
- Pomposelli JJ, Pomfret EA, Burns DL, et al. Life-threatening hypophosphatemia after right hepatic lobectomy for live donor adult liver transplantation. Liver Transpl 2001;7:637-42. [Crossref] [PubMed]
- Tan HP, Madeb R, Kovach SJ, et al. Hypophosphatemia after 95 right-lobe living-donor hepatectomies for liver transplantation is not a significant source of morbidity. Transplantation 2003;76:1085-8. [Crossref] [PubMed]
- Squires MH 3rd, Dann GC, Lad NL, et al. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB (Oxford) 2014;16:884-91. [Crossref] [PubMed]
- Baquerizo A, Anselmo D, Shackleton C, et al. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation 2003;75:2007-14. [Crossref] [PubMed]
- Barak V, Schwartz A, Kalickman I, et al. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med 1998;104:40-7. [Crossref] [PubMed]
- Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta 2007;380:13-23. [Crossref] [PubMed]
- Hill GL, Guinn EJ, Dudrick SJ. Phosphorus distribution in hyperalimentation induced hypophosphatemia. J Surg Res 1976;20:527-31. [Crossref] [PubMed]
- Mashima Y, Ogawa M, Aoki Y, et al. Changes in phosphorus distribution during total parenteral nutrition. JPEN J Parenter Enteral Nutr 1981;5:189-92. [Crossref] [PubMed]
- Lichtman MA, Miller DR, Cohen J, et al. Reduced red cell glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration, and increased hemoglobin-oxygen affinity caused by hypophosphatemia. Ann Intern Med 1971;74:562-8. [Crossref] [PubMed]
- Knochel JP. The clinical status of hypophosphatemia: an update. N Engl J Med 1985;313:447-9. [Crossref] [PubMed]
- Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg 2005;241:343-8. [Crossref] [PubMed]
- Nafidi O, Lepage R, Lapointe RW, et al. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg 2007;245:1000-2. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]