Ophthalmological side effects of interferon therapy of chronic hepatitis C
Introduction
Egypt has the highest prevalence of hepatitis C virus (HCV) in the world, estimated nationally at 14.7% (1). Combined therapy of PEGylated interferon alpha (PEG IFN-α) and ribavirin (RBV) is the base for HCV treatment (2). Ophthalmological complications are amongst many side effects of IFN based therapy for HCV infection (3). The exact mechanism of IFN induced retinopathy is not known, although some investigators have suggested, it may be related to a disrupted retinal microcirculation (4), others believe that IFN impairs the vascular endothelial functions (5).
Methods
In this retrospective non-randomized study, IFN-induced ophthalmological complications were evaluated in 100 patients with chronic HCV who were candidates for IFN based therapy (PEG-IFN and RBV); All cases were selected consecutively from patients enrolled in the national program for treatment of HCV, they were examined and followed up in EL-Kahera El-Fatemya Hospital, (one of the viral hepatitis treatment centers of the national committee for control of viral hepatitis, Ministry of health). They were 83 males and 17 females, their ages ranged between 21–60 years with a mean of 42±10 years. All patients completed the course of therapy (48 weeks).
Exclusion criteria
Other causes of liver disease, decompensated liver disease, HCC, patients with (uncontrolled DM, uncontrolled HTN, or with other significant medical illness such as cardiovascular disease or renal failure) and patients with hypersensitivity to IFN or RBV.
Ocular exclusion criteria
Patients with moderate, severe or very severe non-proliferative diabetic retinopathy (NPDR), or with proliferative diabetic retinopathy (PDR), dense cataract or corneal opacity, iridocyclitis, grade 3 to 4 hypertensive vascular retinopathy, glaucoma, on medications of known ocular side effects, age-related macular degeneration, history of other retinal vascular disorders such as branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO).
This study was approved by the Department Ethics Committee and a signed written informed consent was taken from all participating patients before starting treatment.
All patients were subjected to: thorough history taking, clinical, and ophthalmological examinations including best corrected visual acuity and dilated fundus examination.
Investigations done
- Complete blood picture (CBC);
- Liver biochemical profile (LBP): transaminases; aspartate aminotransferase (AST), and alanine aminotransferase (ALT), alkaline phosphatase (ALP), serum albumin, total bilirubin, INR;
- Kidney function tests (blood urea and serum creatinine);
- Fasting and 2 hours post prandial blood glucose;
- Alpha fetoprotein (AFP), antinuclear antibody (ANA), thyroid stimulating hormone (TSH);
- Hepatitis seromarkers for HCV (anti HCV) and for hepatitis B virus (HBV); (HBsAg, anti HBc and anti HBs) using ELISA technique;
- HCV RNA tested by PCR nested quantitative by IU/mL;
- Rectal snip to diagnose active Schistosomiasis;
- ECG (men over 40, women over 50). Patients were globally evaluated by Child Pugh score (6);
- Abdominal ultrasonography by (Philips iU22 xMatrix ultrasound system). Splenomegaly is considered if >12 cm in females and >13 cm in males (7);
- Histopathological examination by ultrasound guided liver biopsy (in patients with INR <1.4 and platelet count ≥60.000/mm3) according to METAVIR scoring system (8).
Patients were followed up during antiviral therapy (for 48 weeks), clinically by regular symptoms checklist, and by laboratory testing: (I) CBC done every 4 weeks for 24 weeks then at the end of treatment; (II) PCR for HCV RNA at start of therapy, 12, 24, 48 and 72 weeks of therapy.
Follow up ophthalmological investigations
Colored Fundus photography, best corrected visual acuity, slit lamp microscopic examinations of the anterior segment, fundus florescence angiography (FFA) (if retinal involvement is suspected by fundus examination), visual evoked potential (VEP) (if optic nerve dysfunction is suspected by fundus examination) and color vision test (if optic nerve dysfunction is suspected by fundus examination). Ophthalmic examinations were done at start of therapy and at 4, 12, 24, 48 and 72 weeks of therapy.
Statistical analysis
Statistics were done by computer using Epi-Info. A word processing data base and statistics program.
- X mean, SD standard deviation: to measure the central tendency of data and the distribution of data around their mean;
- Independent sample test to compare between 2 groups;
- F’analysis of variance (or ANOVA test) to test statistical significant difference between more than 2 means i.e., difference between 3 or more groups at the same time;
- Person correlation coefficient test ®: to test for linear relation between 2 numeric variables;
- Fisher’s exact test is used to calculate an exact P value for a 2×2 frequency table with small number of expected frequencies, for which the Chi-square test is not appropriate. Significant result is considered if P less than 0.05. Highly significant result is considered if P less than 0.005. Very high significant result is considered if P less than 0.001. (SPSS Inc., Chicago, IL, USA).
Results
The present study was conducted on one hundred HCV naïve patients. They presented to EL-Kahera El-Fatemeya Hospital, Cairo, seeking antiviral therapy according to the program supported by the national committee for control of viral Hepatitis C under supervision by Egyptian Ministry of health.
Demographic features of the studied patients showed that there was male predominance with eighty three males (83%), 36% of them were smokers. Their ages ranged from 21–60 years old with a mean of 42±10 years.
Five (5%) patients had controlled HTN, 7 (7%) patients had controlled DM, 6 (6%) patients had combined HTN and DM and only one (1%) patient with past history of mild bronchial asthma. Of the diabetic patients, 5 patients were on insulin, 6 on oral hypoglycemic drugs and 2 on diets only. They had strict blood sugar monitoring and blood sugar levels were well controlled.
Baseline laboratory data of the studied patients showed that the mean Hb was (14.47±1.58 gm/dL), the mean platelets count was (212±54×103/mm3) and the mean serum albumin was (4.3±0.36 gm/dL). Mean bilirubin was (0.7785±0.2779 mg/dL), AFP (6.0209±9.7493 ngms/dL) and HCV RNA by quantitative PCR (50,964,049±2,530,000,000 IU/mL). ALT and AST were mildly elevated (<2 folds rise).
Fifty two patients received PEG IFN-α 2a (pegasys) and 48 received PEG IFN-α 2b (peg-intron). The dose of PEG IFN-α 2a was 180 mcg (subcutaneously) every week. The mean dose of PEG IFN-α 2b was 1.5 mcg/kg (subcutaneously) every week. The mean starting dose of RBV was (800-1,600 mg) (orally divided dose—twice daily).
All Patients completed the course of 48 weeks of antiviral treatment, and the IFN dose was not reduced in all of them. The end of treatment response (ETR) was achieved in 37/100 (37%) patients and 63/100 (63%) patients failed to achieve ETR.
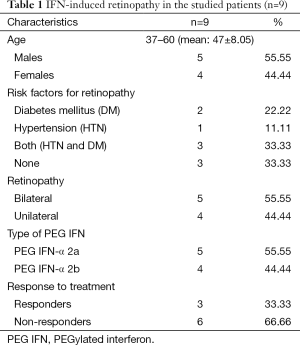
In the studied patients, IFN-induced retinopathy was diagnosed in 9 (9%) patients during the course of antiviral treatment, they included 5 (55.55%) males and 4 (44.44%) females, 5 (55.5%) of them had bilateral lesions, and 4 (44.4%) had unilateral lesions. Three (33.3%) patients were responders and 6 (66.6%) were non responders. Five Patients had received PEG IFN-α 2a and 4 had received PEG IFN-α 2b (Table 1).
Full table
Along the course of treatment, 7/9 (77.77%) patients were detected at week 12 and only 2/9 (22.22%) patients were detected at week 24. With no significant statistical difference (W12–W24, P=0.169 and W12–W48, P=0.114).
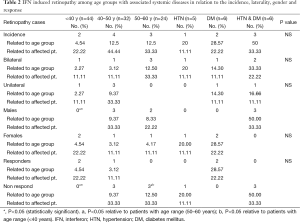
By studying the IFN induced retinopathy among different age groups in relation to the incidence rate within each age group, laterality, gender and the treatment response; it was revealed that incidence rate of retinopathy was marked in patients with advanced age, (4/9; 44.44%) among age range (40–50 years) followed by (3/9; 33.3%) among the age range (50–60 years). Also, the retinopathy signs were bilateral in (3/9; 33.33%) of cases with age range 50–60 years compared to (1/9; 11.11%) among patients aged <40 years and 40–50 years. Five males were affected, 3 of them in age group (40–50 years) and it was statistically significant relative to age group (<40 years). Six patients with IFN-induced retinopathy were non-responders, all of them with advanced age (>40 years) and with statistically significant difference between different age groups (Table 2).
Full table
IFN induced retinopathy with associated systemic diseases in relation to the incidence rate, laterality, gender and treatment response revealed that the patients associated with other systemic risk factors (DM and or HTN) had a higher incidence rate of retinopathy of (6/9; 66.66%), among them 4 patients had bilateral lesions with equal sex distribution. No significant statistical difference was detected between them (Table 2).
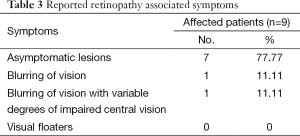
The retinopathy associated symptoms showed that most of the affected patients were asymptomatic (7/9; 77.77%) and the reported symptoms were reversible. The two symptomatic patients showed that one had periods of blurred vision (1/9; 11.11%), and the other patient suffered from various degrees of impairment of his central vision, so there was no need to discontinue or reduce the dose of IFN-RBV therapy. Just RBV dose was reduced in 6 patients in week 24 due to anemia (Table 3).
Full table
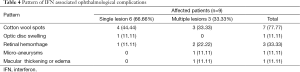
Retinopathy associated signs showed that six cases had single lesion (4 cotton wool spots, one flame shaped hemorrhage and one disc swelling), and 3 cases had multiple lesions (cotton wool spots with micro aneurysms, cotton wool spots with flame shaped hemorrhage and macular edema and the third case showed cotton wool spots with flame shaped hemorrhage). The retinopathy in the 9 patients were reversible by ophthalmologic examination during the course of therapy and at week 72, this proves that retinopathy was not related to DM or HTN (Table 4).
Full table
Discussion
HCV infection is one of the main causes of chronic liver disease worldwide (9). Egypt has the highest prevalence of HCV worldwide and is considered a major cause of chronic hepatitis, liver cirrhosis, HCC, and liver transplantation in the country (10).
IFN-induced retinopathy was first recognized in 1990 when Ikebe and associates (11) reported a 39-years-old patient with retinal hemorrhages and cotton wool spots following intravenous administration of IFN. Reported incidence of IFN-induced retinopathy varied from 18% to 86% according to the country, genotype, and study design. Higher frequencies are usually found in Japan where values of more than 50% are common (3).
The exact mechanism of IFN induced retinopathy is not known, although some investigators have suggested deposition of immune complex at the vessels and immunological dysfunction, others believe that it is related to IFN-induced neuro-visual toxicity (12).
This study was conducted to evaluate IFN-induced ophthalmological complications as regard the incidence, possible associated risk factors and to assess the need to screen for these complications.
The current study included one hundred patients with chronic HCV who received IFN based therapy (PEG IFN-α 2a or PEG IFN-α 2b) and RBV. The mean age of our patients was 42±10 years, our result was in agreement with Cuthbertson et al. (13) whose mean age was 44±10.5. In our study, a significant male predominance was reported; this was also reported by Sherif et al. (14) and Mabrouk et al. (15), which may be due to more male patients attendance.
Few studies have evaluated the efficacy of IFN therapy in persons with persistently normal serum ALT (PNALT). ALT has been reported to increase during IFN therapy and this was apparently one major reason why the NIH consensus development conference recommended that such patients not to be treated with IFN (16). In our study, transaminases were mildly elevated (<2 folds rise). Transaminases are the first biochemical abnormality detected in patients with liver disease, its degree of elevation may correlate with extent of the liver injury but it is not of prognostic value (17). Treatment is widely recommended for patients with elevated ALT (18).
In this study, the incidence of IFN-induced retinopathy in the whole studied patients was found to be (9/100; 9%). Studies by Jain et al. (19), Cuthbertson et al. (13) and Okuse et al. (20) have shown a relatively higher incidence of retinopathy (symptomatic or asymptomatic) ranging from 19% to 64% during treatment with IFN; however Malik et al. (21) and Panetta et al. (22) showed relatively lower incidence of retinopathy (0% and 3.8%, respectively). Also, the estimated prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy in USA was 28.5% and 4.4% respectively, among persons with diabetes aged 40 years and older. While the prevalence nationwide was 3.8% and 0.6% (23).
In our study, we found 5 (55.5%) patients with bilateral lesions and 4 patients had unilateral lesions, which is higher than that reported by d’Alteroche et al. (24) who showed that the lesions were bilateral in 26% of cases.
Also, we found that 6 patients of IFN-induced retinopathy were non-responders (failed to achieve ETR) which was similar to that reported by Fouad et al. (25).
Although Hauser et al. (26) showed that PEG INF-α 2a was associated with a higher sustained virological response in serum than with PEG IFN-α 2b, yet the clinical consequences of PEG IFN-α 2a versus PEG IFN-α 2b are unknown.
Bruno et al. (27) showed that the concentration of PEG IFN-α 2b did not remain stable over the week as a whole. At the end of the week, serum IFN could not be detected in most patients treated with PEG IFN-α 2b. When IFN was no longer detectable in the serum, the viral load increased until the next IFN injection. This phenomenon reduces the efficacy of the drug. While the reduced clearance of PEG IFN-α 2a, provides measurable therapeutic plasma levels even at the end of the weekly dosing period (28). These differences between the two types of PEG IFNs provide better compliance and more safety of PEG IFN-α 2a (29).
These results were in agreement with Wang et al. (30) as they reported that anterior ischemic optic neuropathy (AION) may occur with the use of combined PEG IFN-α 2b and RBV for chronic infection, and patients should be informed about its possible occurrence .
On the contrary, our study showed that IFN-induced retinopathy was reported in 5 patients treated with PEG IFN-α 2a and 4 patients with PEG IFN-α 2b with no detected significant difference.
During the course of IFN treatment, 7 patients developed retinopathy at W12 and only 2 experienced retinopathy at W24, which was similar to that reported by Sherif et al. (14) who stated that the time of appearance of retinopathy signs (between W4 and 16) in about 80% of cases.
Higher incidence of retinopathy was found, in our study, in patients above 40 years. Chronic hypertension predisposes patients to IFN-induced retinopathy through thickening of the walls of the arteries and small arterioles (31). The fact that hypertensive retinopathy induces the formation of flame-shaped hemorrhages and white cotton-wool spots, which are also seen in IFN-induced retinopathy, implies that systemic hypertension and IFN-induced retinopathy may be related to each other (32).
In this study, higher incidence of retinopathy was associated with other systemic risk factors (DM and/or HTN) with tendency to be bilateral. These findings were similar to previous results like Cuthbertson et al. (13), d’Alteroche et al. (24) and Sene et al. (33) who showed that hypertension, diabetes and older age were possible risk factors for IFN-induced retinopathy. On the contrary, Kawano et al. (34), Saito et al. (32) and Okuse et al. (20), reported no association between diabetes and IFN-RBV induced retinopathy, this may be explained by the very low number of patients with DM in those studies.
In our retinopathy cases, six had single lesions and 3 had multiple lesions. The commonest finding was cotton wool spots, which appeared in 7/9 of the patients with retinopathy.
These findings were in agreement with Schulman et al. (35), Cuthbertson et al. (13) and Chuman et al. (36) who reported that the incidence of the cotton wool spots and retinal hemorrhages, retinal micro-aneurysms were reported as a complication of IFN therapy.
Nagaoka et al. (37) classified the IFN-induced retinopathy into two groups depending on the severity, the first group (mild retinopathy); patients with fewer than four hemorrhages, cotton wool spots, or both during treatment, and the second group (severe retinopathy); patients with more than five lesions. In our study, our affected patients were classified as having mild retinopathy.
Most of the affected patients, in our study, were asymptomatic with reversible symptoms in the other two at the end of treatment. One patient had periods of blurred vision, and the other suffered from various degrees of impairment of his central vision, so there was no need to discontinue IFN-RBV therapy. This is in accordance with Mantel et al. (38) and Hayasaka et al. (39) who stated that in spite of the presence of retinopathy, the full course of treatment should be completed.
We excluded, in our study, the possibility that retinopathy observed in patients associated with systemic diseases (DM and or HTN) was caused only by these disorders by the following facts: we included only patients with tight control of blood sugar and ABP, the mean duration of DM was 3 years, and of HTN was 6.5 years, none of the patients had retinopathy signs at the base-line fundal examination before start of INF-RBV therapy, the appearance of retinopathy during the first few months after initiation of IFN therapy, the majority of reported signs have resolved spontaneously during the course of IFN therapy, the IFN retinopathy signs differed as regard their natural course from the classical courses of diabetic or hypertensive retinopathies.
We also excluded the possibility of retinopathy caused by INF-RBV associated anemia, as the mean Hb level of patients with retinopathy at W12 (most common point of retinopathy appearance) was 11.07 g/dL. Anemia can cause retinopathy when Hb decreases below 7 gm/dL (40).
Our results could not be applied to INF based therapy in multiple sclerosis or other conditions as the dose and duration of treatment differs and INF could be combined with other immune modulators, so studying the ophthalmologic side effects in these cohorts of patients should be done. Also, studying retinopathy in INF plus DAA therapy in HCV should be pursued.
Finally, in the light of our results; we have reported that, close follow up of patients with retinopathy (in cooperation with the ophthalmologist) was very important, also, d’Alteroche et al. (24), Sene et al. (33) and Mousa et al. (41) recommended ophthalmological monitoring during IFN therapy.
Although our study had a relatively small sample size but conclusions can be applied generally as INF based therapy of HCV is globally standardized and the side effects of INF should be essentially similar. However, the differences in the incidence among studies could be due to differences in age, background diseases or cause of liver disease in participating patients. And although INF induced retinopathy is not rare but fortunately it's asymptomatic and reversible.
Acknowledgements
We express our deep gratitude to El Fatemya Hospital, for their generous cooperation, also, Dr. Wafaa El Akel, the professor of Endemic Medicine and Hepatogastroenterology, Faculty of Medicine, Cairo University for her support in patients’ enrollment.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Department Ethics Committee and a signed written informed consent was taken from all participating patients before starting treatment.
References
- Mohamoud YA, Mumtaz GR, Suzanne R, et al. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013;13:288. [Crossref] [PubMed]
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82. [Crossref] [PubMed]
- Sauer A, Lenoble P, Bader P, et al. Ocular complications of hepatitis C treatment. J Fr Ophtalmol 2007;30:e20. [PubMed]
- Nishiwaki H, Ogura Y, Miyamoto K, et al. Interferon alfa induces leukocyte capillary trapping in rat retinal microcirculation. Arch Ophthalmol 1996;114:726-30. [Crossref] [PubMed]
- Takase B, Uehata A, Fujioka T, et al. Endothelial dysfunction and decreased exercise tolerance in interferon-alpha therapy in chronic hepatitis C: relation between exercise hyperemia and endothelial function. Clin Cardiol 2001;24:286-90. [Crossref] [PubMed]
- Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. [Crossref] [PubMed]
- Tamayo SG, Rickman LS, Mathews WC, et al. Examiner dependence on physical diagnostic tests for the detection of splenomegaly: a prospective study with multiple observers. J Gen Intern Med 1993;8:69-75. [Crossref] [PubMed]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245-64. [Crossref] [PubMed]
- Egyptian Ministry of Health Annual Report. 2007 Available online: http://www.mohp.gov.eg/Main.asp
- Ikebe T, Nakatsuka K, Goto M, et al. A case of retinopathy induced by intravenous administration of interferon. Folia Opthalmol 1990;41:2291-6.
- Miyamoto K, Suda T, Motokura M, et al. Retinopathy in interferon alpha treatment. J Eye 1993;10:497-500. (Atarashii Ganka).
- Cuthbertson FM, Davies M, McKibbin M. Is screening for interferon retinopathy in hepatitis C justified? Br J Ophthalmol 2004;88:1518-20. [Crossref] [PubMed]
- Sherif AE, Mostafa A, Hanan A. Retinal Complications of Chronic Hepatitis C Treatment. Tropical Medicineand Hygiene, Ophthalmology andMicrobiology Departments, Tanta Faculty of Medicine, Tanta University, Egypt. 2008. Available online: http://knol.google.com/k/retinal-complications-of-chronichepatitis-c-treatment
- Mabrouk M, El-Raziky M, Zayed N, et al. Clinical, biochemical and pathological profiles of 5464 Egyptian chronic hepatitis C-infected patients. Hepatogastroenterology 2013;60:1731-5. [PubMed]
- National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology 2002;36:S3-20. [Crossref] [PubMed]
- Moseley RH. Evaluation of abnormal liver function tests. Med Clin North Am 1996;80:887-906. [Crossref] [PubMed]
- Ghany MG, Strader DB, Thomas DL, et al. American Association for the Study of liver Diseases. Diagnosis, management and treatment of hepatitis C: an update. Hepatology 2009;49:1335-74. [Crossref] [PubMed]
- Jain K, Lam WC, Waheeb S, et al. Retinopathy in chronic hepatitis C patients during interferon treatment with ribavirin. Br J Ophthalmol 2001;85:1171-3. [Crossref] [PubMed]
- Okuse C, Yotsuyanagi H, Nagase Y, et al. Risk factors for retinopathy associated with interferon alpha-2b and ribavirin combination therapy in patients with chronic hepatitis C. World J Gastroenterol 2006;12:3756-9. [Crossref] [PubMed]
- Malik NN, Sheth HG, Ackerman N, et al. A prospective study of change in visual function in patients treated with pegylated interferon alpha for hepatitis C in the UK. Br J Ophthalmol 2008;92:256-8. [Crossref] [PubMed]
- Panetta JD. Gilanin. Interferon-induced retinopathy and its risk in patients with diabetes and hypertension undergoing treatment for chronic hepatitis C virus infection. Aliment Pharmacol Ther 2009;30:597-602. [Crossref] [PubMed]
- Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304:649-56. [Crossref] [PubMed]
- d'Alteroche L, Majzoub S, Lecuyer AI, et al. Ophthalmologic side effects during alpha-interferon therapy for viral hepatitis. J Hepatol 2006;44:56-61. [Crossref] [PubMed]
- Fouad YM, Khalaf H, Ibraheem H, et al. Incidence and risk factors of retinopathy in Egyptian patients with chronic hepatitis C virus treated with pegylated interferon plus ribavirin. Int J Infect Dis 2012;16:e67-71. [Crossref] [PubMed]
- Hauser G, Awad T, Thorlund K, et al. Peginterferon alpha-2a versus peginterferon alpha-2b for chronic hepatitis C. Cochrane Database Syst Rev 2014;2:CD005642. [PubMed]
- Bruno R, Sacchi P, Scagnolari C, et al. Pharmacodynamics of peginterferon alpha-2a and peginterferon alpha-2b in interferon-naïve patients with chronic hepatitis C: a randomized, controlled study. Aliment Pharmacol Ther 2007;26:369-76. [Crossref] [PubMed]
- Foster GR. Review article: pegylated interferons: chemical and clinical differences. Aliment Pharmacol Ther 2004;20:825-30. [Crossref] [PubMed]
- Singal AK, Jampana SC, Anand BS. Peginterferon alfa-2a is superior to peginterferon alfa-2b in the treatment of naive patients with hepatitis C virus infection: meta-analysis of randomized controlled trials. Dig Dis Sci 2011;56:2221-6. [Crossref] [PubMed]
- Wei IH, Wei YH, Woung LC, et al. Anterior ischemic optic neuropathy associated with pegylated interferon therapy for chronic hepatitis C. Ocul Immunol Inflamm 2009;17:191-4. [Crossref] [PubMed]
- Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999;150:263-70. [Crossref] [PubMed]
- Saito H, Ebinuma H, Nagata H, et al. Interferon-associated retinopathy in a uniform regimen of natural interferon-alpha therapy for chronic hepatitis C. Liver 2001;21:192-7. [Crossref] [PubMed]
- Sene D, Touitou V, Bodaghi B, et al. Intraocular complications of IFN-alpha and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol 2007;13:3137-40. [PubMed]
- Kawano T, Shigehira M, Uto H, et al. Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol 1996;91:309-13. [PubMed]
- Schulman JA, Liang C, Kooragayala LM, et al. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology 2003;110:437-42. [Crossref] [PubMed]
- Chuman T, Nao-i N, Sawada A, et al. Interferon-induced retinal changes. Nippon Ganka Gakkai Zasshi 1994;98:616-21. [PubMed]
- Nagaoka T, Yoshida A. Noninvasive evaluation of wall shear stress on retinal microcirculation in humans. Invest Ophthalmol Vis Sci 2006;47:1113-9. [Crossref] [PubMed]
- Mantel I, Konstantinidis L, Zografos L. Interferon-associated retinopathy--a case report. Klin Monbl Augenheilkd 2007;224:350-2. [Crossref] [PubMed]
- Hayasaka S, Fujii M, Yamamoto Y, et al. Retinopathy and sub-conjunctival hemorrhage in patient with chronic viral hepatitis receiving interferon alfa. Br J Ophthalmol 1995;79:150-2. [Crossref] [PubMed]
- Holt JM, Gordon SE. Retinal abnormalities in diseases of the blood. Br J Ophthalmol 1969;53:145-60. [Crossref] [PubMed]
- Mousa N, Basheer T, Gady Y, et al. Is combination therapy interferon and ribavirin in patients with chronic hepatitis C infection toxic for eyes? J Ocul Pharmacol Ther 2013;29:345-8. [Crossref] [PubMed]


