Detection of carcinogenic etheno-DNA adducts in children and adolescents with non-alcoholic steatohepatitis (NASH)
Introduction
Nonalcoholic fatty liver disease (NAFLD) is frequently associated with obesity, insulin resistance and other features of the metabolic syndrome (1-3). While fatty liver per se is a benign condition, the occurrence of non-alcoholic steatohepatitis (NASH) is of major concern since it may progress to fibrosis and cirrhosis of the liver (4) and finally to hepatocellular carcinoma (HCC) (5). It is noteworthy that NASH not only occurs in adults but also in adolescents and children (6,7). When present at young age, it may be a risk factor for the occurrence of HCC later in life. Oxidative stress, excess generation of reactive oxygen species (ROS) and lipid peroxidation (LPO) products in the liver may be of particular importance for the disease onset (8).
In NASH, an induction of hepatic cytochrome P-450 2E1 (CYP2E1) has been described (9,10) which promotes oxidative stress, protein modifications, inflammation, and results in the generation of ROS causing LPO and subsequent cellular damage (11). Among the multiple LPO-products generated, 4-hydroxynonenal (4-HNE) a major product binds to proteins and to DNA via reactive intermediates forming carcinogenic etheno-DNA adducts such as 1,N6-etheno-2'-deoxyadenosine (εdA) (12-14). Both in cultured CYP2E1 overexpressing HepG2 cells and in liver biopsies from patients with alcoholic liver disease (ALD) a significant positive correlation between CYP2E1 induction and the generation of etheno-DNA adducts have been observed (15). Recently, we have detected εdA adducts in the livers of adult NASH patients (10). Since NASH also occurs in children, we investigated whether these carcinogenic DNA lesions also occur already at young age.
Methods
Patients
Twenty-one children/adolescents (13 males, 8 females) aged between 8 and 17 years were liver biopsied. They were recruited from the outpatient Clinic of the university Children Hospital Heidelberg which they visited because of elevated liver enzymes. A complete laboratory work up was performed and infectious, metabolic and autoimmune diseases were excluded. Clinical and metabolic data, including gamma-glutamyl transpeptidase (GGT), aspartate transaminase (AST), and alanine transaminase (ALT)-activities are given in Table 1. None of the patients had diabetes mellitus or consumed alcohol. Besides one patient (Asian) all were of Caucasian ethnicity.
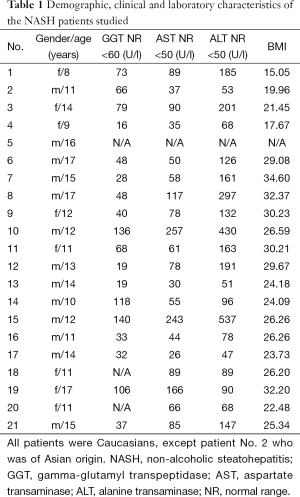
Full table
Histologically normal liver sections from 3 healthy adult subjects were analyzed as controls for εdA background staining intensity. (These biopsies were originally taken to rule out hepatic tumours.) The study was approved in accordance with the declaration of Helsinki by the Ethical Committee of the University of Heidelberg, Germany (Ethic Approval Number: S-510-2009), and the parents of all subjects enrolled gave written informed consent for the participation in the present study.
All biopsies were assessed by histopathologists experienced in liver pathology (CF,PS) and diagnosed as NASH. Both investigators were blinded for immunohistopathology examinations related to the present study. Hepatic inflammation and fibrosis were assessed using the scoring system of Kleinert et al. (16). The inflammation score identifies lobular inflammation with 0-3 (0, no inflammatory foci; 1, <2 foci/200×; 2, 2-4 foci/200×; and 3, >4 foci/200×).
Immunohistochemical detection of etheno-DNA adducts
Staining was performed on liver tissue sections using the method developed in our laboratory (17-20). Paraffin-fixed slides were dipped in phosphate buffered saline (PBS) for 10 min and then placed in 0.3% H2O2 in absolute methanol for 10 min to quench endogenous peroxidase. Slides were incubated with proteinase K (20 mg/mL) (Roche, Mannheim, Germany) in double distilled H2O at room temperature for 10 min to remove histone and non-histone proteins from DNA increasing antibody accessibility. After washing with PBS, slides were treated with 20 µg/mL RNase (Roche, Mannheim, Germany) (heated for 10 min at 80 °C to inactivate DNase) at 37 °C for 1 hour to prevent antibody binding to RNA adducts and then washed in PBS. To denature DNA, cells were treated with 4N HCI for 5 min at room temperature and subsequently rinsed in double distilled water and PBS. The pH was neutralized with 50 mM Trisbase buffer, pH 7.4, for 5 min at room temperature. Non-specific binding sites were blocked with 8% bovine serum albumin (BSA), 2% normal horse serum, 0.05% Tween 20 and 0.05% Triton X-100 for 20 min at 37 °C. Slides were incubated at 4 °C overnight with the primary monoclonal antibody EM-A-1 against εdA (provided by Drs. P. Lorenz and M. Rajewksy, University of Essen, Essen, Germany), at a dilution of 1:20 (20) and 2% normal horse serum to block nonspecific binding. After washing with PBS, the antibody detection was performed using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol (incubation with secondary antibody: horse anti-mouse IgG (H + L) 1:400 for 40 min at room temperature). Diaminobenzidine (DAB) was used as a chromogen to visualize the reaction. The reaction was stopped after 5 min with H2O. Slides were counterstained with 4',6'-diamidino-2-phenylindole and mounted with Roti-Histokitt II (Carl Roth, Karlsruhe, Germany). All slides were subjected to the same standard conditions. Negative controls were performed by omitting the primary antibody.
Immunohistochemical staining of CYP2E1 and protein bound 4-HNE
Paraffin-embedded liver biopsy samples were cut into 6 mm sections and placed on 3-aminopropyl-triethoxysilan-coated glass slides. Sections were treated with 0.5% H2O2 in absolute methanol for 10 min to quench endogenous peroxidase activity. Thereafter, sections were incubated at room temperature for 2 hours with the primary antibody (rabbit anti-human CYP2E1, 1:400, Chemicon, Hofheim, Germany) or with rabbit anti-4-HNE (1:250, Alexis, Lörrach, Germany) and 5% normal goat serum to block nonspecific binding. Vectastain Elite ABC (Vector Laboratories, Burlingame, CA, USA) was used for detection according to the manufacturer’s protocol. Staining was developed by incubating the sections for 5 min in DAB. Sections were counterstained with hematoxylin and mounted with Roti-Histokitt II (Carl Roth, Karlsruhe, Germany). Negative controls were performed by omitting the primary antibody.
Immunohistochemical detection of alkyladenine DNA glycosylase (AAG) expression
AAG expression was determined in 6 biopsies. Paraffin-embedded tissue was dewaxed in xylene, rehydrated in a decreasing ethanol series and finally washed in PBS. Antigen retrieval was performed by heating the slides in citrate buffer (10 mM, pH 6 with 0.05% Tween-20) for 10 minutes. After cooling down to room temperature, the slides were treated with 0.3% H2O2 in absolute methanol for 20 minutes to quench endogenous peroxidise activity. Thereafter, sections were incubated overnight at 4 °C with the primary antibody (mouse antihuman APNG, 1:50, Santa Cruz, CA, USA) and 2% normal horse serum to block nonspecific binding. VECTASTAIN Elite ABC kit was used for detection according to the manufacturer’s protocol (secondary antibody: anti-mouse species 1:200 and 2% normal horse serum in PBS). Staining was developed by incubating the sections for 5 minutes with DAB. After dehydrating the sections in an increasing alcohol series, they were counterstained with hematoxilin and mounted in Roti-Histokitt II (Carl Roth GmbH, Karlsruhe, Germany) mounting medium. Negative controls were performed by omitting the primary antibody.
Imaging and semi-quantitative analyses of etheno-DNA adducts, CYP2E1 expression and protein-bound 4-HNE
After immunohistochemical staining for εdA, CYP2E1 and 4-HNE, the blinded slides were independently scored by a second investigator. Representative pictures were taken at a magnification of 100×/400× with a SPOT FLEX system (Model 15.2 64 Mp, shifting pixel, DIAGNOSTIC Instruments, Inc., Sterling Heights, Michigan, USA, SPOT VERSION 4.6.4.69) and analyzed using Image J software (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/).
Staining intensity for CYP2E1 and 4-HNE was assessed according to the scale devised by Tsutsumi et al. (21), whereby 3+, 2+, 1+ and 0 denote intense, moderate, slight and no specific immunostaining, respectively. Staining intensity of εdA was estimated and recorded as 1 to 4. These 4 graded levels were used as there is always a very low background staining for εdA which was assigned as grade 1. In addition; the frequency of nuclei positively stained for εdA was calculated as % of stained cell nuclei over a total number of cells counted.
Statistics
The empirical distribution of continuous parameter was reported with mean, minimum, and maximum, with absolute and relative frequency by categorical variables. Spearman rank correlation was calculated to examine possible correlation between characteristics. A value of α=0.05 was used as level of significance.
Results
Figure 1 shows the various grades of immunohistochemical staining for εdA in cell nuclei of hepatocytes in liver biopsies from NASH children. Among 21 biopsies, εdA levels in the liver were high in 3 (14.3%), moderate in 5 (23.8%), weak in 9 (42.9%), and not elevated in 4 (19%) individuals showing only a low background level. As biopsies from healthy children cannot be taken for ethical reasons, we used liver sections from healthy adult subjects (biopsy was taken to rule out hepatic tumours) as the only available controls for εdA background staining. A very low background level of etheno adducts (range, 0-27 adducts per 109 parent bases) has been detected in liver DNA from asymptomatic humans and untreated rodents (22) (data not shown).
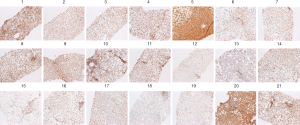
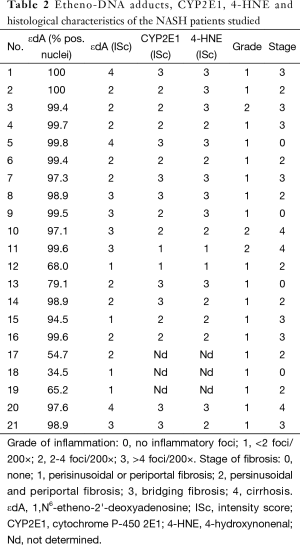
Table 2 summarizes disease grade (inflammatory activity) and stage (degree of fibrosis) as classified by histopathological evaluation (fat, inflammation, fibrosis). Staining intensity of CYP2E1, protein bound 4-HNE and εdA in nuclear DNA is shown.
Full table
A significant correlation between CYP2E1 and protein bound 4-HNE (r=0.60; P=0.008) was found. A trend for a positive relationship between the staining intensity of εdA and CYP2E1 (r=0.45; P=0.06) was apparent but not seen for εdA and protein bound 4-HNE (r=0.39; P=0.11). Neither εdA, protein bound 4-HNE nor CYP2E1 correlated significantly with serum enzyme activities.
A comparison of εdA, CYP2E1, and 4-HNE staining in representative biopsies of 6 children is depicted in Figure 2. In general, strong staining intensity paralleled each other for CYP2E1 and protein-bound 4-HNE, but was less apparent for CYP2E1 vs. εdA, as reflected by the Spearman rank correlation (r=0.45; P=0.061).
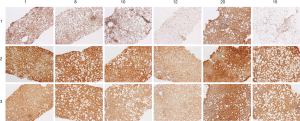
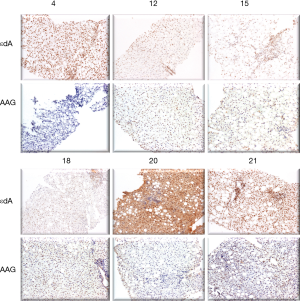
Figure 3 shows AAG expression vs. εdA in 6 biopsies. In 5 out of 6 biopsies the level of etheno adducts seems reversely related to AGG expression.
Inflammatory activity (grading) and degree of fibrosis (staging) correlated significantly in these biopsies (r=0.49; P=0.02). However, a significant correlation between histology, εdA, CYP2E1 or 4-HNE was not observed.
Significant correlations also occurred between serum GGT activity and the activities of AST (r=0.67; P=0.002) as well as of ALT (r=0.49; P=0.037) and between the serum activities of both transaminases (r=0.78; P=0.0001).
Discussion
Our results presented here demonstrate for the first time the occurrence of carcinogenic, LPO-derived DNA adducts in the majority of liver biopsies of young NASH patients. εdA was analyzed, using a highly specific monoclonal antibody (17) for immunohistochemical detection. Etheno-DNA adducts are highly mutagenic lesions producing specific point mutations and other types of genomic lesions in all organisms tested so far (23). Exocyclic DNA adducts are derived from reaction of DNA bases A, C, 5methyl-C, and G with LPO-products that are formed from inflammation-driven oxidative stress reactions (24-26). One of the major LPO-product, 4-HNE induces DNA damage through its reactive intermediates (27), one of which is 2,3-epoxy-4-hydroxynonanal (28). Complex LPO reactions in vivo lead to both substituted (with fatty acid chains attached) such as heptanone-etheno adducts (27,29,30) and to the unsubstituted adducts εdA, 3,N4-etheno-2'-deoxycytosine (εdC), as well as two isomeric etheno-2'-deoxyguanosine adducts (26,31,32). A hitherto unknown LPO-derived DNA adduct formed in vivo, 3,N4-etheno-5-methyl-2'-deoxycytidine was recently detected in human liver that may perturb genome methylation (33).
εdA, a marker lesion for exocyclic adducts is formed by reaction of LPO-products with DNA bases in human (31) and rodent tissues (14). After DNA repair in internal organs, etheno adducts are eliminated and excreted in urine (20).
Strong support for the pathological relevance of etheno adducts is provided by previous studies which found up to 1-2 orders of magnitude elevated adduct levels in human liver of patients with cancer prone inflammatory diseases (31,34).
In liver biopsies from ALD patients, the induction of CYP2E1 by chronic ethanol consumption resulted in excess generation of LPO products measured as protein bound 4-HNE, and etheno-DNA adducts (15). A prominent role of CYP2E1 in hepatocarcinogenesis has been further supported in CYP2E1 overexpressing human cells, and in a rodent NASH model using Zucker rats with and without ethanol ingestion (15,35,36). Thus, in both human and experimental studies, a significant positive correlation between CYP2E1 expression and εdA levels could be consistently demonstrated; moreover, the inhibition of CYP2E1 by clomethiazole resulted in a significant reduction of εdA levels in rat liver (15). CYP2E1 has also been found to be responsible for ethanol-induced oxidative DNA damage in the rodent liver (37) and for the occurrence of hepatic adenomas in chemically induced hepatocarcinogenesis following chronic alcohol ingestion in rats (35). Hepatic CYP2E1 was also induced in adult NASH patients (9,10), regardless whether they have developed diabetes or not (38). It has been shown in ob/ob mice, an experimental model for NASH, that CYP2E1 promotes liver injury (39).
It was demonstrated by Fujita et al. (40) that oxidative stress also plays an important role in disease pathogenesis of NASH. 8-oxodG, a DNA base-modified product generated by hydroxyl radicals, was significantly higher in NASH liver biopsy samples compared to subjects with simple steatosis.
Although in ALD patients CYP2E1 expression and activity did significantly correlate with εdA (15), this was not found in hepatic biopsies from NASH children, where only a trend with borderline significance (r=0.45; P=0.06; N=21) was found. Several explanations for this weak relationship may exist:
- Our sample size is too small;
- Only εdA was determined while correlations may exist with other types of etheno adducts that have been found in human tissues (24,31);
- Most of ROS formation was inflammation driven rather than generated through CYP2E1, since it has been reported that the high prevalence of oxidative stress in children with NAFLD is associated with increased severity of steatohepatitis (41);
- The large inter-individual variations of hepatic εdA adduct levels could be caused by varying rates of their formation (affected by detoxifying enzymes, antioxidants and levels of inflammatory mediators) and by their removal rates by DNA repair enzymes. These modifying factors have not been measured.
To support the latter assumption, a study on the expression of a DNA repair enzyme, AAG in liver biopsies from NASH children was attempted (Figure 3). Unsubstituted etheno-DNA adducts are eliminated mainly by base-excision repair. AAG and the mismatch-specific thymidine-DNA glycosylase remove εdA and εdC respectively (42-44).
Indeed, low AAG staining seemed to parallel a high etheno-DNA adduct formation, suggesting that low (or impaired) base excision repair activity may lead to increased DNA adduct generation. Confirmation of this hypothesis with a larger sample size is mandatory but of great interest in the context of a reduced DNA damage repair in NASH (44). Indeed, in steatotic livers of obese subjects with NASH, a decrease in nucleotide excision repair (NER) was found for M1dG, another major LPO-derived DNA adduct, formed by reaction of malondialdehyde with deoxyguanine and by other pathways. Importantly the observed reduction in NER capacity upon hepatic inflammation in NASH patients was associated with and may be a consequence of reduced damage recognition (45). For an HNE-derived substituted etheno-dG adduct (termed HNE-dG) possibly also generated in NASH liver, NER was shown to be the major repair pathway in human cells (46). Thus, studies whether the hepatic DNA repair enzyme capacities of AAG and NER are reduced, leading to increased DNA damage are highly warranted.
Finally, it is noteworthy that DNA damage is particular detrimental during foetal development. Results from a cohort study of white blood cells (WBC) from mother-newborn child pairs lend support that NASH related oxidative stress is a self perpetuating process, and LOP-derived DNA damage could already be triggered in utero. As shown in 77 WBC-DNA samples from mother-newborn child pairs, highly variable levels of etheno-DNA adducts were detected (47). These results confirm that this DNA damage arises in vivo from LPO-derived reactive aldehydes such as 4-HNE and indicate that a similar signature of DNA damage is found in foetus and mother.
In conclusion, in children and young adults with NASH, one of the mutagenic and carcinogenic etheno-DNA adducts εdA was unequivocally detected in 17 out of 21 liver biopsies. Semi-quantitative analysis of εdA by immunohistochemistry revealed large inter-individual variations. CYP2E1 expression and εdA levels showed only a trend for a positive relationship, possibly because εdA may additionally be formed through ROS generation due to inflammation. Whether hepatic etheno adducts may serve as predictive risk markers in NASH children to develop HCC later in life remains to be investigated.
Acknowledgements
This study was supported by grants of the Dietmar Hopp and Manfred Lautenschläger Foundations to H. K. Seitz. We thank Ms. Heike Grönebaum for typing this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved in accordance with the declaration of Helsinki by the Ethical Committee of the University of Heidelberg, Germany (Ethic Approval Number: S-510-2009), and the parents of all subjects enrolled gave written informed consent for the participation in the present study.
References
- Ruiz-Extremera Á, Carazo Á, Salmerón Á, et al. Factors associated with hepatic steatosis in obese children and adolescents. J Pediatr Gastroenterol Nutr 2011;53:196-201. [PubMed]
- Lerret SM, Garcia-Rodriguez L, Skelton J, et al. Predictors of nonalcoholic steatohepatitis in obese children. Gastroenterol Nurs 2011;34:434-7. [PubMed]
- Pacifico L, Nobili V, Anania C, et al. Pediatric nonalcoholic fatty liver disease, metabolic syndrome and cardiovascular risk. World J Gastroenterol 2011;17:3082-91. [PubMed]
- Fujii H, Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol 2012;47:215-25. [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8. [PubMed]
- Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a “growing” problem? J Hepatol 2007;46:1133-42. [PubMed]
- Schreuder TC, Verwer BJ, van Nieuwkerk CM, et al. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol 2008;14:2474-86. [PubMed]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol 2013;58:395-8. [PubMed]
- Weltman MD, Farrell GC, Hall P, et al. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology 1998;27:128-33. [PubMed]
- Linhart KB, Glassen K, Peccerella T, et al. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr 2015;4:117-23. [PubMed]
- Abdelmegeed MA, Banerjee A, Yoo SH, et al. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol 2012;57:860-6. [PubMed]
- Winter CK, Segall HJ, Haddon WF. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Res 1986;46:5682-6. [PubMed]
- Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis 1996;17:2105-111. [PubMed]
- el Ghissassi F, Barbin A, Nair J, et al. Formation of 1,N6-ethenoadenine and 3,N4-ethenocytosine by lipid peroxidation products and nucleic acid bases. Chem Res Toxicol 1995;8:278-83. [PubMed]
- Wang Y, Millonig G, Nair J, et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009;50:453-61. [PubMed]
- Kleinert DE, Brunt E, Van Natte M, et al. Design and validation of histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [PubMed]
- Eberle G, Barbin A, Laib RJ, et al. 1,N6-etheno-2'-deoxyadenosine and 3,N4-etheno-2'-deoxycytidine detected by monoclonal antibodies in lung and liver DNA of rats exposed to vinyl chloride. Carcinogenesis 1989;10:209-12. [PubMed]
- Yang Y, Nair J, Barbin A, et al. Immunohistochemical detection of 1,N(6)-ethenodeoxyadenosine, a promutagenic DNA adduct, in liver of rats exposed to vinyl chloride or an iron overload. Carcinogenesis 2000;21:777-81. [PubMed]
- Frank A, Seitz HK, Bartsch H, et al. Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis 2004;25:1027-31. [PubMed]
- Nair J, Nair UJ, Sun X, et al. Quantifying etheno-DNA adducts in human tissues, white blood cells, and urine by ultrasensitive (32)P-postlabeling and immunohistochemistry. Methods Mol Biol 2011;682:189-205. [PubMed]
- Tsutsumi M, Lasker JM, Shimizu M, et al. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology 1989;10:437-46. [PubMed]
- Nair J, Barbin A, Guichard Y, et al. 1,N6-ethenodeoxyadenosine and 3,N4-ethenodeoxycytine in liver DNA from humans and untreated rodents detected by immunoaffinity/32P-postlabeling. Carcinogenesis 1995;16:613-7. [PubMed]
- Barbin A. Etheno-adduct-forming chemicals: from mutagenicity testing to tumor mutation spectra. Mutat Res 2000;462:55-69. [PubMed]
- Vaca CE, Wilhelm J, Harms-Ringdahl M. Interaction of lipid peroxidation products with DNA. A review. Mutat Res 1988;195:137-49. [PubMed]
- Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med 1990;8:541-3. [PubMed]
- Sodum RS, Chung FL. 1,N2-ethenodeoxyguanosine as a potential marker for DNA adduct formation by trans-4-hydroxy-2-nonenal. Cancer Res 1988;48:320-3. [PubMed]
- Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem 2008;283:15545-9. [PubMed]
- Sodum RS, Chung FL. Stereoselective formation of in vitro nucleic acid adducts by 2,3-epoxy-4-hydroxynonanal. Cancer Res 1991;51:137-43. [PubMed]
- Medeiros MH. Exocyclic DNA adducts as biomarkers of lipid oxidation and predictors of disease. Challenges in developing sensitive and specific methods for clinical studies. Chem Res Toxicol 2009;22:419-25. [PubMed]
- Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer 2011;128:1999-2009. [PubMed]
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med 2007;43:1109-20. [PubMed]
- Sodum RS, Chung FL. Structural characterization of adducts formed in the reaction of 2,3-epoxy-4-hydroxynonanal with deoxyguanosine. Chem Res Toxicol 1989;2:23-8. [PubMed]
- Nair J, Godschalk RW, Nair U, et al. Identification of 3,N(4)-etheno-5-methyl-2'-deoxycytidine in human DNA: a new modified nucleoside which may perturb genome methylation. Chem Res Toxicol 2012;25:162-9. [PubMed]
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006;391:499-510. [PubMed]
- Ye Q, Lian F, Chavez PR, et al. Cytochrome P450 2E1 inhibition prevents hepatic carcinogenesis induced by diethylnitrosamine in alcohol-fed rats. Hepatobiliary Surg Nutr 2012;1:5-18. [PubMed]
- Tsuchishima M, George J, Shiroeda H, et al. M. Chronic ingestion of ethanol induces hepatocellular carcinoma in mice without additional hepatic insult. Dig Dis Sci 2013;58:1923-33. [PubMed]
- Bradford BU, Kono H, Isayama F, et al. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology 2005;41:336-44. [PubMed]
- Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003;37:544-50. [PubMed]
- Dey A, Cederbaum AI. Induction of cytochrome P450 2E1 promotes liver injury in ob/ob mice. Hepatology 2007;45:1355-65. [PubMed]
- Fujita N, Miyachi H, Tanaka H, et al. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev 2009;18:424-32. [PubMed]
- Nobili V, Parola M, Alisi A, et al. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med 2010;26:471-6. [PubMed]
- Gros L, Ishchenko AA, Saparbaev M. Enzymology of repair of etheno-adducts. Mutat Res 2003;531:219-29. [PubMed]
- Saparbaev M, Kleibl K, Laval J. Escherichia coli, Saccharomyces cerevisiae, rat and human 3-methyladenine DNA glycosylases repair 1,N6-ethenoadenine when present in DNA. Nucleic Acids Res 1995;23:3750-5. [PubMed]
- Hang B, Chenna A, Rao S, et al. 1,N6-ethenoadenine and 3,N4-ethenocytosine are excised by separate human DNA glycosylases. Carcinogenesis 1996;17:155-7. [PubMed]
- Schults MA, Nagle PW, Rensen SS, et al. Decreased nucleotide excision repair in steatotic livers associates with myeloperoxidase-immunoreactivity. Mutat Res 2012;736:75-81. [PubMed]
- Feng Z, Hu W, Amin S, et al. Mutational spectrum and genotoxicity of the major lipid peroxidation product, trans-4-hydroxy-2-nonenal, induced DNA adducts in nucleotide excision repair-proficient and -deficient human cells. Biochemistry 2003;42:7848-54. [PubMed]
- Arab K, Pedersen M, Nair J, et al. Typical signature of DNA damage in white blood cells: a pilot study on etheno adducts in Danish mother-newborn child pairs. Carcinogenesis 2009;30:282-5. [PubMed]


