How much ischemia can the liver tolerate during resection?
Introduction
The Pringle maneuver is eponymously attached to the Australian surgeon James Hogart Pringle, who, while working at the Royal Infirmary in Glasgow, in 1908 first reported occlusion of the portal vein and hepatic artery [i.e., vascular inflow occlusion (VIO)] by compressing the hepatoduodenal ligament to control blood loss in trauma patients with a liver laceration (1). The induction of total liver ischemia is inherent to this technique, and for several decades it was believed it could only be applied for 15-20 min. VIO was therefore not extensively used, until Huguet et al. claimed that ischemia time in non-diseased livers could be extended to 65 min (2,3). As a result, the use of continuous VIO during resection became more popular during the 1980s (4) and in 1987 the application of intermittent VIO was first described by Makuuchi et al. (5). In this procedure, VIO was applied in cycles of 30 min that were followed by 5 min of reperfusion, which could be repeated in cases that necessitated prolonged VIO.
The main aim of VIO is to reduce intraoperative blood loss and the consequent need for blood transfusion (6), which is a risk factor for postoperative mortality and morbidity (7-9). VIO, however, also results in hepatic ischemia/reperfusion (I/R) injury, which refers to the sterile inflammatory response and hepatocellular damage that are triggered when the hepatic blood (i.e., oxygen) supply is restored after a period of ischemia. Insofar as the duration of ischemia correlates positively with the hallmarks of I/R injury [e.g., ATP depletion and oxidative stress (10-12)], animal studies indicate that prolonged ischemia leads to an increased mortality risk (13,14). In addition, livers affected by parenchymal disorders such as (non-)alcoholic fatty liver disease, cirrhosis, and chemotherapy-induced sinusoidal obstruction syndrome are more susceptible to I/R injury and therefore have a lower ischemic tolerance (15,16). As such, the routine use of VIO during liver surgery may be abandoned in the near future. For complex cases that require (on demand) VIO to safely complete parenchymal transection, however, the maximum acceptable duration of hepatic ischemia remains a relevant issue.
In an attempt to address this issue, Gurusamy et al. have reviewed the status quo of VIO during liver surgery (6). It was concluded that VIO effectively reduces intra-operative blood loss and decreases blood transfusion requirements, while no negative effects on post-operative mortality and morbidity rates were noted. The use of VIO during liver resections is therefore generally considered safe. However, since all the studies included used different clamping regimens, no conclusion could be drawn concerning the maximum tolerable ischemia time.
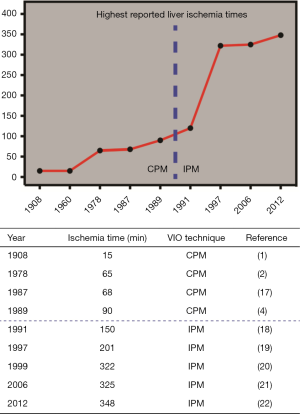
Over the past decades, several reports have challenged the maximum duration of ischemia the liver can tolerate (Figure 1). Man et al. reported a safe upper limit of 120 min of intermittent VIO in 1999 (23), while in 2012 Torzilli et al. claimed that ischemia times exceeding 120 min are well tolerated using this technique (24). In addition, two case reports mention the successful use of exceptionally long durations of liver ischemia: 322 min (20) and 348 min (22), respectively. These reports have reinvigorated the discussion about how much ischemia the liver can actually tolerate.
Nevertheless, the abovementioned reports exclusively cover cases in which the patient was not affected by any type of parenchymal liver disease. This is relevant since several studies indicate that compromised livers poorly tolerate prolonged VIO (25-29). Due to a steep increase in the global prevalence of conditions that underlie parenchymal liver disease such as the metabolic syndrome, VIO is nowadays frequently used in livers with a compromised parenchymal status. Very little data is however available on the effect of prolonged VIO (i.e., >90 min) in this patient category, with only one reported case that describes a cumulative VIO duration of 204 min in a cirrhotic liver (27).
Although several reviews on VIO techniques have been published (30,31), none have focused specifically on the duration of ischemia that the liver can tolerate. In this paper, the relation between parenchymal liver disease and the upper limit of VIO duration is therefore discussed, with specific focus on the use of prolonged (>60 min) ischemia times during liver resection.
VIO techniques
Several techniques to induce VIO during liver surgery have been introduced. Of these, the Pringle maneuver, or hepatic pedicle clamping, is the best known VIO method. A sling is placed around the hepatoduodenal ligament, which comprises both the hepatic artery and the portal vein, and tightened to halt the hepatic blood supply (1). The Pringle maneuver can be used continuously [continuous Pringle maneuver (CPM)] or intermittently [intermittent Pringle maneuver (IPM)]. During IPM, the portal triad is generally occluded for 15-20 min (ischemia) followed by a period of 5-10 min of declamping (reperfusion). Consequently, IPM is applied repeatedly during parenchymal transection.
Another technique for VIO is total hepatic vascular exclusion (THVE) (32). The infrahepatic and suprahepatic vena cava are clamped, as is the portal pedicle, resulting in complete isolation of the liver from the circulation. A similar technique is selective hepatic vascular exclusion (SHVE), also referred to as THVE with preservation of caval flow (33,34). SHVE requires that the liver is disconnected from the vena cava by ligation of the short perforator veins, after which the hepatic veins and the portal pedicle are clamped, thereby inducing hepatic in- and out-flow occlusion with a patent vena cava.
In order to counteract the risks of I/R injury, all techniques for VIO are occasionally combined with ischemic preconditioning (IP). IP is a technique that aims to reduce hepatic I/R injury by inflicting a short ischemic insult followed by a short period of reperfusion prior to a prolonged period of VIO (35,36).
Ischemia times reported using continuous VIO
Continuous VIO is one of the most widely used techniques that aim to reduce blood loss in liver surgery. Multiple studies have been published on continuous vascular occlusion, several of which will be discussed in the following section. The results of these studies are also summarized in Table 1. In 1989, the operative management of 142 cases using continuous VIO (THVE, N=35 or CPM, N=107) was reported (4). Liver failure occurred more in patients with cirrhosis (5/15) compared to patients with a non-diseased liver (4/127, P<0.001). The duration of ischemia (mean ± SEM) was similar in patients with non-diseased livers compared to those with cirrhotic livers [(32.6±1.2) vs. (34.1±4.2) min in N=127 and N=15, respectively]. No differences in mortality and morbidity were found between the VIO <45 min (range, 8-44 min, N=119) and the VIO >45 min (range, 45-90 min, N=23) groups. Intergroup differences in postoperative mortality and morbidity were also not observed in 53 hepatectomies with three groups divided by the duration of VIO [THVE (N=25) or CPM (N=28)] (37). VIO was applied <30 min in group 1 (range, 15-29 min, N=9), 30-60 min in group 2 (N=29), and >60 min in group 3 (range, 60-85 min, N=15). Consequently, it was suggested that the liver could tolerate continuous VIO for >60 min, although no exact maximum duration was specified. When continuous VIO time in 34 patients with uncompromised liver parenchyma was >60 min [THVE (N=15), CPM (N=15) or THVE and CPM sequentially (N=4)] with a mean ± SEM VIO time of 73.6±2.5 min (range, 60-127 min), no correlation between the duration of ischemia and postoperative liver injury [aspartate aminotransferase (AST), alanine aminotransferase (ALT)], liver function (bilirubin, prothrombin time), or postoperative complications was seen (25). Accordingly, it was concluded that CPM could be safely applied for up to 90 min in healthy livers. Another study addressing 26 patients who underwent continuous VIO [CPM (N=14) or THVE (N=12)] with ischemia times exceeding 1 h was published in 1994 (38). The mean ± SEM duration of VIO was 68±7.5 min in patients with non-diseased livers (N=17) and 64.7±6.8 min in patients with compromised livers (chemotherapy-induced liver disease, cirrhosis, or steatosis, N=9). Liver failure was seen in 4 patients with cirrhosis, which was reflected by the finding that postoperative morbidity was higher in diseased livers (77.8% vs. 11.8%, P<0.05). It was therefore concluded that continuous VIO of ≤85 min was not a risk factor in healthy livers, but that diseased livers are more prone to complications after continuous VIO of >60 min. In 26 cirrhotic patients exposed to 50-75 min (group 1, N=14) or 30-42 min (group 2, N=12) of VIO, less blood loss (mean ± SD) was seen compared with cirrhotic patients operated without VIO (group 3, N=21; 819±572, 523±457, and 1,652±1,240 mL blood loss in group 1, 2, and 3, respectively) (39). Although peak postoperative serum ALT levels were higher in group 1 than in groups 2 and 3 (P=0.02), no differences were found in postoperative mortality and morbidity. It was therefore concluded that continuous VIO could be tolerated for about 50 min in cirrhotic livers. However, considering that 12 patients underwent continuous VIO for >50 min, with a maximum of 75 min, cirrhotic livers can possibly withstand the use of CPM for ≤75 min.
Full table
Taken together, these studies show that continuous VIO can be used for a period up to 90 min in uncompromised livers and to at least 50 min in diseased livers without increasing mortality and morbidity rates.
Ischemia times reported using intermittent VIO
It was suggested that intermittent VIO reduces I/R injury and could therefore prolong the tolerable ischemia time (5). Several reports using intermittent VIO in either damaged (i.e., cirrhosis, steatosis, or chronic hepatitis) or uncompromised liver parenchyma are highlighted in the next section and are summarized in Table 2. Elias et al. started to use IPM routinely since 1987 (18). They retrospectively analyzed 20 patients exposed to intermittent VIO of >90 min in cycles of 20 min of ischemia and 5 min of reperfusion (20/5 min cycle). The mean VIO time was 109 min (range, 90-150 min), with a VIO duration of >140 min in two patients. Postoperative complications occurred in 7 patients (28.7%), which is in line with other reports (4,19,40). Total blood loss was the only parameter that positively correlated with prolonged ischemia times. Thus, it was concluded that intermittent VIO might even be safe for ≤150 min. In 100 patients with non-diseased and pre-damaged livers (i.e., due to cirrhosis or chronic hepatitis) randomized between IPM (N=50) or no VIO (N=50), mortality and morbidity rates were comparable (19). The median ischemia time was 88 min (range, 24-201 min). Total blood loss was lower in the VIO group (median, 1,280 mL; range, 90-8,500 mL) compared with the control group (median, 1,990 mL; range, 260-13,900 mL; P<0.001). It was therefore concluded by the authors that IPM is safe and effective in both compromised and uncompromised livers, but that it should not be applied for >120 min. Subsequently, a group of 12 patients who were operated with cumulative ischemia times of >120 min was compared with this cohort (23). The median ischemia time in this additional group was 134.5 min (range, 123-201 min) and 83 min (range, 24-114 min) in the patients that were randomized to IPM (N=50). A tendency towards lower blood loss was observed for <120 min IPM compared to >120 min IPM [(median, 1,010 mL; range, 230-9,020 mL) vs. (median, 2,030 mL; range, 560-9,420 mL), P=0.06)]. No differences were found in terms of mortality and morbidity. Based on these results, it was concluded that IPM can be used safely for 120 min without increasing postoperative mortality and morbidity rates in both non-diseased and diseased livers. IPM of >90 min (15/5 min cycles) was retrospectively evaluated in 34 cases by Ishizaki et al. (21). In group 1 (N=25), cumulative VIO duration was 90-120 min and in group 2 (N=9), cumulative VIO duration was >120 min (range, 120-325 min). There was less blood loss (mean ± SD) in group 1 (883±461 mL) compared with group 2 (1,409±1,039 mL) (P=0.047). Moreover, lower peak transaminase levels (mean ± SD) were noted for group 1 compared with group 2 [AST: (410±324) vs. (966±590) U/L, P=0.001; ALT: (383±350) vs. (913±690) U/L, P=0.006], although peak total bilirubin levels were comparable. Additionally, there were no intergroup differences in postoperative mortality or complications. This was confirmed in 189 patients operated with a cumulative IPM time of >60 min (15/5 min cycles), in which underlying cirrhosis or steatosis was seen in 65 and 83 patients, respectively (24). Patients with ischemia times (mean ± SD) of 60-120 min (group 1, 86±17 min, N=117) were compared to patients with ischemia times of >120 min (group 2, 161±48 min, N=72), ranging from 120 to 348 min. Peak levels of AST, ALT, and total bilirubin were all higher in group 2 (P=0.002, P<0.001, P=0.004, respectively), but mortality and morbidity rates were similar. Consequently, it was proposed that IPM can be used for >120 min when absolutely necessary, with a reported maximum VIO duration of 325 to 348 min (21,24).
Full table
As stated earlier, cirrhotic livers are more susceptible to I/R injury, which limits the maximum VIO duration in these patients (26). Eighty-three patients with cirrhotic livers who did not have ascites, had a serum bilirubin concentration <3.5 mg/dL, and had an indocyanine green clearance rate of less than 40% were divided into three groups: group 1 with <40 min ischemia (N=39), group 2 with 40-80 min of ischemia (N=28), and group 3 with >80 min of ischemia (range, 84-204 min) (N=16) (27). The mean ± SD cumulative ischemia time was 25.5±6.7, 58.3±10.2, and 110.5±34.7 min in group 1, 2, and 3, respectively. In one patient, a VIO time of 204 min was necessary. Operative blood loss and blood transfusion requirements were higher in group 3 compared to group 1 (P<0.001). Peak AST and ALT levels were also higher in group 3 compared with the other study arms (both P<0.001). Nevertheless, mortality and morbidity rates were comparable between all groups. Accordingly, it was concluded that carefully selected patients with cirrhotic livers can safely withstand prolonged IPM of >120 min, possibly up to a maximum of 204 min.
Based on the abovementioned results, prolonged IPM can be safely used beyond 120 min in uncompromised livers with a potential maximum duration of 348 min and in thoroughly selected cirrhotic livers with an apparent upper limit of 204 min.
Ischemia times in continuous versus intermittent VIO
Continuous and intermittent VIO have been extensively studied, but few studies have compared the two techniques directly (summarized in Table 3). In 1999, IPM (20/5 min cycles, group 1, N=44) was compared with CPM (group 2, N=42) in a randomized clinical trial with a mean duration of VIO of 46 min (range, 20-118 min) and 41 min (range, 16-67 min) in group 1 and 2, respectively (28). Postoperative liver injury markers were similar in both groups, but the correlation between elevation of serum ALT levels and duration of VIO was stronger in group 2 (Pearson’s r=0.68, P<0.001) than in group 1 (Pearson’s r=0.38, P<0.01). This finding suggests that the liver tolerates IPM better than CPM. The overall incidence of postoperative complications was comparable between both groups (30% in group 1 vs. 26% in group 2) although a trend was noted towards a higher incidence of acute liver failure in group 2 (4 patients) vs. group 1 (0 patients, P=0.05). All patients who developed acute liver failure had pre-existent liver disease (cirrhosis or steatosis). Therefore, 3 subgroups were compared with respect to the use of IPM and CPM: patients with healthy livers (group 1, N=50), patients with steatotic livers (group 2, N=11, >20% steatosis), and patients with cirrhotic livers (group 3, N=25). In group 2, the lowest peak prothrombin time was seen following IPM. The use of CPM resulted in significantly higher serum ALT levels in groups 2 and 3 compared with IPM (both P<0.05). Higher bilirubin levels in group 3 were found for CPM compared to IPM (P<0.05). The authors therefore concluded that IPM is superior to CPM in terms of parenchymal tolerance to ischemia, especially when underlying liver disease was present. This was however not confirmed in a study with 35 cirrhotic patients comparing IPM (15/5 min cycles) (group 1, N=17) with CPM (group 2, N=18) (29). Only patients aged <75 years with hepatocellular carcinoma and Child Pugh Score A were included. The mean ± SD VIO time was 40.4±1.7 min (range, 20-65 min) and 35.5±13.9 min (range, 16-84 min) in group 1 and 2, respectively. Postoperative complications and mortality were similar (P=0.2 and P=0.1, respectively). When VIO duration was compared (<30 vs. >30 min) instead of VIO technique, patients with >30 min developed more complications (N=8 vs. N=0, P=0.02). No differences were found in postoperative AST, ALT, prothrombin time, or bilirubin levels. Because approximately 75% of the patients did not receive blood transfusions, it was concluded that both techniques were effective in reducing blood loss and that there was no difference in the severity of hepatic I/R injury. This fueled the discussion that IPM might not be necessary in the cirrhotic liver for ischemia times of up to 60 min.
Full table
The effects of prolonged IPM and CPM on hepatic I/R injury were also investigated in two animal models (results are summarized in Table 4) (41,42). In swine, 120 min of IPM (12/3 min cycles) was better tolerated than 120 min of CPM (41). Sinusoidal endothelial cell function, reflected by the ability to clear hyaluronic acid from the circulation, was superior in the IPM group. Corroboratively, the extent of hepatocellular necrosis at 6 h of reperfusion was higher in the CPM group. Similar results were obtained in rat models of IPM and CPM (42). Three VIO regimens were compared: IPM in 15/5 min cycle (group 1), IPM in 30/5 min cycle (group 2), and CPM (group 3). Three different cumulative ischemia times were compared: 60, 90, and 120 min. Survival rates were similar up to 90 min of ischemia. When VIO time was prolonged to 120 min, however, survival was better using IPM (70%, 70%, and 20% in group 1, 2, and 3 respectively, group 1 or group 2 vs. group 3, P<0.05). Serum AST and ALT levels were significantly lower on post-operative day 1 following IPM, irrespective of the employed IPM regimen, compared with CPM for both 90 and 120 min of ischemia (P<0.05 for group 1 or group 2 vs. group 3). There were no intergroup differences noted when VIO periods of 60 min were compared.

Full table
Although the differences are generally small and mostly seen in serum transaminase levels in contrast to mortality and morbidity rates, IPM seems to be better tolerated than CPM in both uncompromised and compromised livers, especially when prolonged ischemia times (>60 min) are necessary.
Ischemia times with IP followed by continuous VIO
IP was first clinically tested by Clavien et al. in 1999 in 24 patients undergoing major hepatectomy (35). After that, it became a topic of interest in liver surgery, of which several studies are discussed (also see Table 5). Using a standardized CPM regimen of 30 min, Clavien et al. found lower post-operative serum ALT and AST levels in the 12 patients who received IP with CPM (IP-CPM) compared with the 12 patients who received CPM alone (35). This effect was even more pronounced in a small subgroup of patients with steatosis. In a subsequent randomized trial, 100 patients were randomized between IP-CPM (group 1, N=50) or CPM alone (group 2, N=50) (43). Peak ALT and AST levels were lower in group 1 (406 vs. 519 U/L and 364 vs. 520 U/L, P=0.049 and P=0.028, respectively), although mortality and morbidity rates were comparable. Ischemia times of <60 min were associated with better outcomes in group 1. Based on a small subgroup analysis (N=13), it was additionally claimed that steatotic livers benefited more from IP than healthy livers. This was evidenced by the considerable reduction in peak transaminase levels seen in fatty livers treated with IP compared to those subjected to CPM alone (363 vs. 602 U/L, respectively, P=0.049). Guided by these results, IP could be mostly beneficial in ischemia times of ≤60 min. Another study found lower serum AST levels on postoperative day 1 in patients operated with IP-CPM (N=21) than in patients operated with CPM only (N=21), despite the fact that these patients were subjected to longer ischemia times [(54±19) vs. (36±14) min in IP-CPM and CPM, respectively, P=0.001] (44). It is unclear whether the 10 min of IP were added to the cumulative duration of ischemia, but despite this, IP imparted a protective effect given the lower serum AST levels found. One study comparing IP followed by SHVE (group 1, N=30) to SHVE only (group 2, N=30) during major hepatectomies with similar VIO durations [44.5±9.2 min (group 1) and 47.7±8.3 min (group 2), P=0.2], found comparable peak post-operative (mean ± SD, group 1 vs. group 2) serum AST [(851±1,733) vs. (427±166) U/L, P=0.2], ALT [(717±995) vs. (403±200) U/L, P=0.1], and bilirubin [(63.0±60.0) vs. (81.2±71.0) µmol/L, P=0.3] levels, as were the severity and number of complications (45). Better clinical outcomes were seen, however, in a randomized controlled trial comparing IP-CPM (group 1, N=30) with CPM alone (group 2, N=31) (46). Specifically, there was reduced (mean ± SD) blood loss [(1,280±910) vs. (1,940±760) mL, P=0.001], a lower transfusion incidence (17% vs. 48% of patients, P=0.006), and a lower complication rate (20% vs. 45% overall complications, P=0.04) in group 1. Serum ALT and bilirubin levels did not differ between the two groups during the first postoperative week. In a trial in which 84 patients were randomly assigned to IP (10 min ischemia, 15 min of reperfusion) followed by SHVE (group 1, N=41) or SHVE alone (group 2, N=43), post-operative (day 1; mean ± SD, group 1 vs. group 2) levels of AST [(288±140) vs. (498±255) U/L, P<0.05] as well as the cytokines IL-6 [(177±88) vs. (325±198) pg/dL, P<0.05] and IL-8 [(219±112) vs. (369±187) pg/dL, P<0.05] levels were lower in group 1 (47). Mean ± SD VIO duration was similar in group 1 (42±11 min) and group 2 (42±10 min), implying that IP prior to SHVE effectively attenuated I/R injury. In liver biopsies taken at 1 h of reperfusion, the number of apoptotic cells was lower in group 1, further highlighting the protective effect of IP.
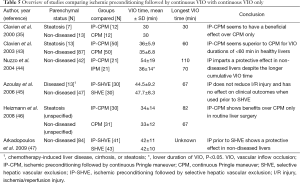
Full table
All studies discussed above applied IP prior to a period of continuous VIO of <60 min. The protective effect of IP before prolonged (>60 min) ischemia has only been investigated in animal models (summarized in Table 6) (13,48,49). One study assigned 24 pigs to undergo partial liver resection (65%) with IP (10 min ischemia, 10 min reperfusion) followed by 90 (N=6, group 1) or 120 (N=6, group 2) min of CPM either 90 (N=6, group 3) or 120 (N=6, group 4) min IPM only (48). Plasma AST and oxidative stress metabolite (i.e., malondialdehyde) levels were lower when IP followed by CPM was compared to IPM only following 90 min of ischemia. However, there was no intergroup difference with respect to the extent of hepatocellular necrosis. When the ischemic insult was extended to 120 min, IPM proved superior to IP-CPM in terms of AST release, plasma malondialdehyde levels, and histological necrosis score. IP-CPM therefore appears more beneficial when the VIO duration does not exceed 90 min, whereas IPM seems preferred when VIO is extended to 120 min. The same conclusions were drawn based on a mouse model comparing IP followed by CPM (group 1), IPM only (group 2), and CPM only (group 3) for VIO times of 75 and 120 min (13). IP-CPM is protective up to 75 min, which was evidenced by the 100% survival 3 days after surgery in groups 1 and 2 vs. 0% survival in group 3. When ischemia times were extended to 120 min, a survival rate of only 14% was seen in group 1 vs. 71.4% in group 2, and 0% in group 3, indicating that IPM offers the best results when prolonged VIO is required. These results are supported by a study by Seyama et al. (49), in which the severity of hepatic I/R injury was evaluated as a function of five different VIO regimens in rats. Three groups underwent different cycles of IPM [4 15/5 min cycles (group 1), 6 10/3.3 min cycles (group 2), or 12 5/1.7 min cycles (group 3)]. In addition, group 4 received 10 min of IP followed by 60 min CPM and group 5 was subjected to 60 min CPM only. The IPM groups all showed lower ALT levels and less hepatocellular necrosis at 3 h of reperfusion compared with the CPM groups (groups 4 and 5). There were no differences in liver injury when individually comparing the 2 CPM groups (groups 4 and 5) or the 3 IPM groups (groups 1-3). IPM therefore seems better tolerated by the liver than IP-CPM or CPM alone when ischemia times exceed 60 min.
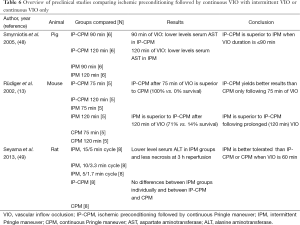
Full table
Taken together, IP-CPM seems to aggravate I/R injury when ischemic intervals of more than 75 min are used. However, IP may improve post-operative outcomes when applied before a shorter (<75 min) period of continuous VIO.
Ischemia times in IP followed by intermittent VIO
In addition to IP before CPM, two clinical studies also compared the effect of IP (10 min ischemia, 10 min reperfusion) followed by IPM (IP-IPM) to IPM alone (also see Table 7) (50,51). One study randomly assigned 84 patients to either IP-IPM (group 1) or IPM (group 2) (50). Ischemia times (mean ± SD) were similar in both study arms (45.0±19.6 and 52.4±27.7 min, respectively). Moreover, there were no differences in the number as well as the severity of postoperative complications or hepatocellular injury markers (e.g., ALT). The second study evaluated the clinical feasibility of IP-IPM (51), postulating that the additional 20 min operating time inherent to IP should be avoided when the therapeutic efficacy of IP-IPM is subpar. Thirty-two patients were therefore divided into 2 experimental groups (N=16/group) based on the planned resection (i.e., major or minor liver resection). Thereafter, each group was randomly divided into 2 groups, receiving either IP-IPM or IPM alone, resulting in 4 groups (N=8/study arm). Microdialysis analysis showed that IP-IPM reduced the hepatic glycogenic activity and lactate formation during and directly after surgery, suggesting that IP alleviated the ischemia-induced metabolic perturbations seen in the IPM-only groups. However, since clinical outcome parameters such as serum liver injury markers (AST, ALT), serum liver function markers (bilirubin, prothrombin time), and postoperative complications were similar amongst all experimental groups, the therapeutic value of IP-IPM remains questionable.

Full table
Discussion
Hepatic I/R injury is still a main concern in liver surgery and a balance between blood loss and I/R injury must be established for every liver resection. VIO effectively reduces blood loss (6) yet induces I/R injury when used for prolonged periods (10,11,14,52,53). In light of this critical trade-off, there is still uncertainty on the maximal duration of VIO that the liver can withstand. This debate is sparked by multiple reports on the safe use of ischemia times of >300 min (20,22,24).
Cirrhotic livers seem to benefit more from IPM. Wu et al. (27) performed liver resections in cirrhotic livers using intermittent VIO up to 204 min, whereas Kim et al. (39) reported a maximum of 75 min of continuous VIO in cirrhotic patients. Despite that Capussotti et al. (29) did not find any differences in clinical outcomes between intermittent and continuous VIO in cirrhotic patients, evidence from animal studies suggests that intermittent VIO in a 15/5 min cycle provides the best protection against hepatocellular injury when the total ischemia time is 60 min (54).
Intermittent VIO has a complication rate that is comparable to continuous VIO (28). The highest complication rates are seen with >60 min of ischemia. Studies presenting data using continuous VIO report a complication rate of 53-56% (25,37). When IPM was used, complications were seen in 20-65% of patients (18,21,24). A recent systematic review, however, indicated that the complication rate was similar for intermittent and continuous VIO vs. no VIO (6). However, no comparison was made between studies with ischemia times of >60 or <60 min, so a meta-analysis should be performed to assess whether intermittent or continuous VIO is preferred for prolonged ischemia times.
Another possibility to decrease I/R injury is IP, which was first described by Clavien et al. (35) and was shown to attenuate surgery-induced liver injury in several randomized clinical trials (43,46). In spite of these beneficial effects, combining IP with VIO durations of >60 min seems to be hazardous (13,48) and it is therefore advised to only use IP when parenchymal transection is expected to last <60 min.
Diseased (e.g., cirrhotic or steatotic) livers seem to benefit more from IP than livers with uncompromised parenchyma (55). The ischemia times used in the latter study, however, were extremely short (<20 min). In cirrhotic mice, a protective effect was noted for ischemia times of up to 60 min compared with CPM alone (56). IP has a protective effect before CPM in pre-damaged livers, but should not be used when ischemia times are >60 min.
VIO might lose ground in liver surgery, as some large centers reported using the Pringle maneuver in only 17% of liver resections since 1999 as well as a 35% decrease in its use compared to before 1999 (57). Although 17% is exceptionally low when compared with other studies performed in the last decade (58-60), the routine use of VIO may be omitted in the future. One should, however, note that bleeding complications can occur, in which case the use of VIO is an important tool in order to regain hemodynamic control. VIO will therefore always have a role in liver surgery, although one should always be aware of the consequences of prolonged ischemia. Further evaluation of the pathophysiology of I/R injury and its consequences therefore remains important (61,62), as well as the development of better interventional strategies (53).
There moreover seem to be no strict limitations regarding the duration of ischemia in healthy livers, for ischemia times of more than 300 min have been reported (20-22). Considering that only 3 patients have been exposed to VIO durations of such caliber, it is however difficult to draw definitive conclusions from these reports. These results are nevertheless promising in view of the ongoing progress in hepatic surgery. Furthermore, these data should be kept in mind when complex hepatic resections are planned and one should not withhold immediately when ischemia times of >120 min are expected during hepatectomies.
To establish the upper limit of hepatic ischemia, more data should be obtained from prolonged ischemia periods in the clinical setting. Since most hepatectomies can be performed within 30-40 min of VIO, it is not feasible to derive these data from randomized controlled trials. The maximum duration of VIO will therefore likely be determined based on case reports and small retrospective studies.
Overall conclusions
Prolonged (≥60 min) hepatic VIO (38) can be safely applied using both continuous and intermittent VIO regimens. The latter showed a benefit in terms of intra-operative blood loss and blood transfusion requirements, but did not reduce mortality and morbidity rates (6). Intermittent VIO can safely be applied for >120 min in healthy livers and may even be extended to 300 min when absolutely necessary. In well-selected cirrhotic livers, a cumulative ischemia time of 120 min is considered safe, with an upper limit of at least 200 min. Considering that most parenchymal transections can be completed within 30-40 min, clamping of the hepatic pedicle therefore does not appear to cause additional harm to the liver remnant.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-9. [PubMed]
- Huguet C, Nordlinger B, Bloch P, et al. Tolerance of the human liver to prolonged normothermic ischemia. A biological study of 20 patients submitted to extensive hepatectomy. Arch Surg 1978;113:1448-51. [PubMed]
- Huguet C, Nordlinger B, Galopin J, et al. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet 1978;147:689-93. [PubMed]
- Delva E, Camus Y, Nordlinger B, et al. Vascular Occlusion for Liver Resections. Operative management and tolerance to hepatic ischemia: 142 Cases. Ann Surg 1989;209:211-8. [PubMed]
- Makuuchi M, Mori T, Gunvén P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet 1987;164:155-8. [PubMed]
- Gurusamy KS, Kumar Y, Ramamoorthy R, et al. Vascular occlusion for elective liver resections. Cochrane Database Syst Rev 2009.CD007530. [PubMed]
- Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg 1999;23:676-80. [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406. [PubMed]
- Sitzmann JV, Greene PS. Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann Surg 1994;219:13-7. [PubMed]
- van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev 2012;23:69-84. [PubMed]
- Reiniers MJ, van Golen RF, van Gulik TM, et al. Reactive Oxygen and Nitrogen Species in Steatotic Hepatocytes: A Molecular Perspective on the Pathophysiology of Ischemia-Reperfusion Injury in the Fatty Liver. Antioxid Redox Signal 2014;21:1119-42. [PubMed]
- van Golen RF, Reiniers MJ, Vrisekoop N, et al. The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury. Antioxid Redox Signal 2014;21:1098-118. [PubMed]
- Rüdiger HA, Kang KJ, Sindram D, et al. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg 2002;235:400-7. [PubMed]
- van Golen RF, Reiniers MJ, Heger M, et al. Solutions to the discrepancies in the extent of liver damage following ischemia/reperfusion in standard mouse models. J Hepatol 2015;62:975-7. [PubMed]
- Veteläinen R, van Vliet A, Gouma DJ, et al. Steatosis as a risk factor in liver surgery. Ann Surg 2007;245:20-30. [PubMed]
- Kim YI. Ischemia-reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg 2003;10:195-9. [PubMed]
- Delva E, Camus Y, Paugam C, et al. Hemodynamic Effects of Portal Triad Clamping in Humans. Anesth Analg 1987;66:864-8. [PubMed]
- Elias D, Desruennes E, Lasser P. Prolonged intermittent clamping of the portal triad during hepatectomy. Br J Surg 1991;78:42-4. [PubMed]
- Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-11. [PubMed]
- Sakamoto Y, Makuuchi M, Takayama T, et al. Pringle maneuver lasting 322 min. Hepatogastroenterology 1999;46:457-8. [PubMed]
- Ishizaki Y, Yoshimoto J, Miwa K, et al. Safety of prolonged intermittent pringle maneuver during hepatic resection. Arch Surg 2006;141:649-53. [PubMed]
- Procopio F, Torzilli G. Forty-nine colorectal cancer liver metastases in one-stage hepatectomy with cumulative Pringle time lasting 348 min. Updates Surg 2012;64:241-3. [PubMed]
- Man K, Fan ST, Ng IO, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg 1999;134:533-9. [PubMed]
- Torzilli G, Procopio F, Donadon M, et al. Safety of intermittent Pringle maneuver cumulative time exceeding 120 minutes in liver resection: a further step in favor of the “radical but conservative” policy. Ann Surg 2012;255:270-80. [PubMed]
- Hannoun L, Borie D, Delva E, et al. Liver resection with normothermic ischemia exceeding 1 h. Br J Surg 1993;80:1161-5. [PubMed]
- Nishimura T, Nakahara M, Kobayashi S, et al. Ischemic injury in cirrhotic livers: an experimental study of the temporary arrest of hepatic circulation. J Surg Res 1992;53:227-33. [PubMed]
- Wu CC, Hwang CR, Liu TJ, et al. Effects and limitations of prolonged intermittent ischaemia for hepatic resection of the cirrhotic liver. Br J Surg 1996;83:121-4. [PubMed]
- Belghiti J, Noun R, Malafosse R, et al. Continuous Versus Intermittent Portal Triad Clamping for Liver Resection. Ann Surg 1999;229:369-75. [PubMed]
- Capussotti L, Nuzzo G, Polastri R, et al. Continuous versus intermittent portal triad clamping during hepatectomy in cirrhosis. Results of a prospective, randomized clinical trial. Hepatogastroenterology 2003;50:1073-7. [PubMed]
- van Gulik TM, de Graaf W, Dinant S, et al. Vascular occlusion techniques during liver resection. Dig Surg 2007;24:274-81. [PubMed]
- Lau WY, Lai EC, Lau SH. Methods of vascular control technique during liver resection: a comprehensive review. Hepatobiliary Pancreat Dis Int 2010;9:473-81. [PubMed]
- Huguet C, Addario-Chieco P, Gavelli A, et al. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg 1992;163:602-5. [PubMed]
- Smyrniotis V, Farantos C, Kostopanagiotou G, et al. Vascular control during hepatectomy: review of methods and results. World J Surg 2005;29:1384-96. [PubMed]
- Cherqui D, Malassagne B, Colau P, et al. Hepatic Vascular Exclusion With Preservation of the Caval Flow for Liver Resections. Ann Surg 1999;230:24-30. [PubMed]
- Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155-62. [PubMed]
- Boyko VV, Pisetska ME, Tyshchenko OM, et al. Role of ischemic preconditioning in hepatic ischemia-reperfusion injury. Hepatobiliary Surg Nutr 2014;3:179-84. [PubMed]
- Huguet C, Gavelli A, Addario Chieco P, et al. Liver ischemia for hepatic resection: where is the limit? Surgery 1992;111:251-9. [PubMed]
- Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg 1994;178:454-8. [PubMed]
- Kim YI, Nakashima K, Tada I, et al. Prolonged normothermic ischaemia of human cirrhotic liver during hepatectomy: a preliminary report. Br J Surg 1993;80:1566-70. [PubMed]
- Capussotti L, Muratore A, Ferrero A, et al. Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg 2006;93:685-9. [PubMed]
- van Wagensveld BA, van Gulik TM, Gelderblom HC, et al. Prolonged continuous or intermittent vascular inflow occlusion during hemihepatectomy in pigs. Ann Surg 1999;229:376-84. [PubMed]
- Chiappa A, Makuuchi M, Zbar A, et al. Comparison of continuous versus intermittent hepatic pedicle clamping in an experimental model. Hepatogastroenterology 2001;48:1416-20. [PubMed]
- Clavien PA, Selzner M, Rüdiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg 2003;238:843-50. [PubMed]
- Nuzzo G, Giuliante F, Vellone M, et al. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl 2004;10:S53-7. [PubMed]
- Azoulay D, Lucidi V, Andreani P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg 2006;202:203-11. [PubMed]
- Heizmann O, Loehe F, Volk A, et al. Ischemic preconditioning improves postopeartive outcome after liver resections: A randomized controlled study. Eur J Med Res 2008;13:79-86. [PubMed]
- Arkadopoulos N, Kostopanagiotou G, Theodoraki K, et al. Ischemic preconditioning confers antiapoptotic protection during major hepatectomies performed under combined inflow and outflow exclusion of the liver. A randomized clinical trial. World J Surg 2009;33:1909-15. [PubMed]
- Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. Ischemic preconditioning versus intermittent vascular inflow control during major liver resection in pigs. World J Surg 2005;29:930-4. [PubMed]
- Seyama Y, Imamura H, Inagaki Y, et al. Intermittent clamping is superior to ischemic preconditioning and its effect is more marked with shorter clamping cycles in the rat liver. J Gastroenterol 2013;48:115-24. [PubMed]
- Scatton O, Zalinski S, Jegou D, et al. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg 2011;98:1236-43. [PubMed]
- Winbladh A, Björnsson B, Trulsson L, et al. Ischemic preconditioning prior to intermittent Pringle maneuver in liver resections. J Hepatobiliary Pancreat Sci 2012;19:159-70. [PubMed]
- Bhogal RH, Curbishley SM, Weston CJ, et al. Reactive Oxygen Species Mediate Human Hepatocyte Injury During Hypoxia / Reoxygenation. Liver transpl 2010;16:1303-13. [PubMed]
- van Golen RF, Reiniers MJ, Olthof PB, et al. Sterile inflammation in hepatic ischemia/reperfusion injury: present concepts and potential therapeutics. J Gastroenterol Hepatol 2013;28:394-400. [PubMed]
- Lei DX, Peng CH, Peng SY, et al. Safe upper limit of intermittent hepatic inflow occlusion for liver resection in cirrhotic rats. World J Gastroenterol 2001;7:713-7. [PubMed]
- Li SQ, Liang LJ, Huang JF, et al. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol 2004;10:2580-4. [PubMed]
- Jang JH, Kang KJ, Kang Y, et al. Ischemic preconditioning and intermittent clamping confer protection against ischemic injury in the cirrhotic mouse liver. Liver Transpl 2008;14:980-8. [PubMed]
- Fan ST, Mau Lo C, Poon RTP, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg 2011;253:745-58. [PubMed]
- Weiss MJ, Ito H, Araujo RLC, et al. Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol 2013;20:285-94. [PubMed]
- Millet G, Truant S, Leteurtre E, et al. Volumetric analysis of remnant liver regeneration after major hepatectomy in bevacizumab-treated patients: a case-matched study in 82 patients. Ann Surg 2012;256:755-61; discussion 761-2. [PubMed]
- Nobili C, Marzano E, Oussoultzoglou E, et al. Multivariate analysis of risk factors for pulmonary complications after hepatic resection. Ann Surg 2012;255:540-50. [PubMed]
- van Golen RF, Reiniers MJ, van Gulik TM, et al. Organ cooling in liver transplantation and resection: how low should we go? Hepatology 2015;61:395-9. [PubMed]
- Heger M, Reiniers MJ, van Golen R. Mitochondrial Metabolomics Unravel the Primordial Trigger of Ischemia/Reperfusion Injury. Gastroenterology 2015;148:1071-3. [PubMed]