Laparoscopic liver resection for posterosuperior and anterolateral lesions—a comparison experience in an Asian centre
Introduction
Minimally invasive surgery has been one of the recent developments in liver surgery after the first laparoscopic liver resection (LLR) was reported in 1992 (1). It was initially performed for benign and cystic lesions in easily accessible locations. As surgical techniques, experience and technology improved over the past few decades, laparoscopic liver surgery has been applied to malignant lesions, major resections and even tumors in difficult locations without compromising safety or oncologic principles. In 2000, Cherqui et al. reported the first feasibility series of 30 LLR (2). The slow and cautious adoption of LLR has been attributed to the complexity of the procedures, safety issues such as risk of bleeding and concerns of oncologic compromise (3). In 2008, an international group of experts established the Louisville statement stating recommendations for LLR, including favorable indications such a solitary peripheral lesions in liver segments 2 to 6, less than or equal to 5 cm; laparoscopic resection of posterior and/or superior segments was not accepted as standard of care (4). However, with the rapid advancement of laparoscopic equipment, techniques and accumulation of experience, LLR has become more established for extended indications. Recently, the 2nd international consensus on LLR was held in Iwate, Japan. The guidelines evolved to state that LLR can be appropriate even in difficult segments [posterosuperior (PS) segments 1, 4a, 7, 8], albeit in well-selected patients and in experienced hands.
Cirrhosis is associated with higher mortality and morbidity rates in open liver surgery (5-8). The laparoscopic approach has advantages probably due to its inherent minimally invasive nature rendering a lower physiologic stress response. This advantage may be more pronounced in cirrhotic patients, thus achieving lower mortality and morbidity rates when compared to cirrhotic patients who undergone open liver surgery (9). However, the effect of cirrhosis coupled with difficult tumor locations in LLR is not well studied.
We evaluate our experience with LLR, comparing the outcomes for tumors located in the PS segments of the liver with tumors located in the anterolateral lesions (AL) segments of the liver in a patient population with significant proportion being cirrhotic.
Methods
A retrospective review of all LLR performed at the Department of General Surgery and the Department of Hepatopancreatobiliary and Transplant Surgery, Singapore General Hospital, between January 1, 2006 and January 15, 2015 was conducted. Institutional review board approval was obtained prior. Patients were selected from a prospectively maintained database and were included if they had a LLR with or without other organ resections. PS segments are defined as segments 1, 4a, 7, and 8; anterolateral segments are the remaining segments (2, 3, 4b, 5, 6) (10-12). The PS and AL groups were defined based on the location of the tumors on preoperative imaging. Clinical parameters examined included age, gender, American Society of Anesthesiologists (ASA) score, Child-Pugh score, tumour location, size and number. Surgical parameters such as surgical approach, reasons for conversion, operative time, estimated blood loss (EBL) and blood transfusion were recorded. Pathological data such as margin status, resection margin, presence of cirrhosis and tumor type were reviewed. Postoperative outcomes including morbidity, its grade, mortality and the length of hospital stay (LOS) were also included in the analysis. All data were obtained from a prospective computerized clinical database (Sunrise Clinical Manager version 5.8, Eclipsys Corporation, Atlanta, Georgia, USA) and operative data were obtained from a prospective computerized operative database (OTM 10, IBM, Armonk, New York, USA). These data were verified or supplemented by patient chart reviews when necessary.
Postoperative morbidity and mortality were defined as complications or deaths within 90 days after surgery, respectively. Postoperative morbidity was classified according to the Clavien-Dindo classification (13). Major resections were defined using the International Hepato-Pancreato-Biliary Association (IHPBA) Brisbane classification—those consisting of three or more Couinaud liver segments (14).
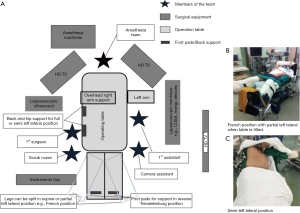
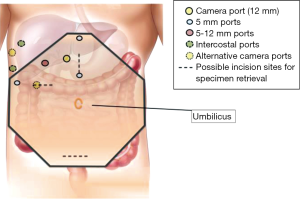
Surgical techniques for LLR at our institution have been previously described (11). Our approach and operative set-up for right-sided PS lesions are further detailed here. The procedure was performed with the patient placed in partial, semi- or full-left lateral position with or without the legs apart and in some degree of reverse Trendelenburg (Figure 1). Patient positioning, where the surgeon stands, and trocar placement was individualised to the tumor location and patient’s constitution. Open technique insertion of the 1st port (camera) was performed in the right paramedian supraumbilical region, ensuring that the tip of the camera was able to reach over the dome of the liver. After insufflation to 12 mmHg of pneumoperitoneum, evaluation of the abdominal wall in the area of the intended trocar placement was performed as every individual’s abdomen insufflates differently to a certain degree. Two-to-three working trocars (5/12 and 5 mm) were placed in the right lateral region and below the costal arch in a gentle curve from the right anterior axillary line to the midline; these are placed at least 5 cm away from the initial camera port site and from each other to minimise intracorporeal interference. An additional 1-2 trocars (5 mm) are inserted in the epigastrium, or along the midline or left paramedian, for application of the Pringles manoeuvre (short profile 5 mm trocar) and an assistant port for retraction of the right lobe medially or for suction and irrigation. Transthoracic intercostal ports are placed when deemed necessary (Figure 2). We found the flexible laparoscope (HD EndoEYE, Olympus, Japan) useful for looking over the dome of the liver and around corners especially in the resection of PS lesions. After cholecystectomy and lowering of the hilar plate, dissection was performed to isolate the right and left Glisson’s pedicles at the inferior surface of the quadrate lobe. For a right posterior sectionectomy, the right Glisson’s pedicle was further dissected to define the anterior and posterior pedicles. This is followed by an extraparenchymal isolation and division en masse using a linear stapler of the right Glisson’s pedicle for a right hemihepatectomy or the right posterior Glisson’s pedicle for a right posterior sectionectomy, if possible. If extraparenchymal isolation of the pedicle proves to be difficult, intraparenchymal glissonian approach can be another option. Ischemic demarcation is visualised and marked out, margins and lesion are then confirmed with the laparoscopic ultrasound. The liver is then fully mobilized laterally and inferiorly from the retrohepatic inferior vena cava. Parenchymal transection was performed using a variety and combination of instruments usually involving Cavitron Ultrasonic Surgical Aspirator (CUSA) (Valleylab, Boulder, CO, USA) and an advanced energy device as previously described (11).
The perioperative outcomes were compared between two groups (PS and AL). Continuous, normally distributed variables are presented as the mean ± standard deviation, and categorical variables are expressed as the median (range). Fisher’s exact test or Pearson’s Chi-square test was applied to compare proportions between groups as appropriate. For comparison of continuous variables, the Mann-Whitney U test was used. Statistical analyses were performed with SPSS version 16.0 for Windows (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA).
Results
Preoperative demographic data and indications for LLR
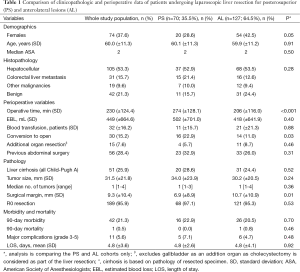
The clinicopathologic characteristics of patients are summarized in Table 1. Mean patient age was 60±11.3 years. There were 74 (37.6%) females and 51 (25.9%) patients had underlying liver cirrhosis (all Child-Pugh’s A). Patients’ age, ASA, and the severity of underlying liver disease were similar between groups (P>0.05), although there were proportionally more females in the AL group (P=0.05). The indications for the resection were hepatocellular carcinoma (HCC) (n=105; 53%), colorectal cancer liver metastases (CRLM) (n=31; 16%), and benign pathology (n=42; 21%) (Table 1). The indications for resection were similar in the two groups (P=0.28). There were no differences in tumor characteristics, including mean tumor size and number of tumors between the two groups (P=0.24 and 0.36, respectively).
Full table
Intraoperative and postoperative outcome
Out of the 197 consecutive LLRs, 30 patients (15.2%) underwent conversion to an open procedure. There were significantly more conversions in the PS group than the AL group (22.9% vs. 11%; P=0.03). The most common reason for conversion was intraoperative bleeding that was difficult to safely control laparoscopically. Operative time was also longer in the PS group (274 vs. 206 min; P<0.001). Otherwise, perioperative outcomes, including blood loss, patients requiring blood transfusion, hospital stay, morbidity and mortality were similar between the two groups (P>0.05) (Table 1).
Indications for surgery and pathology
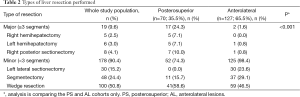
There were no differences in the severity of the underlying liver disease, or in the histologic parameters, including the tumor type, number and size (P>0.05) between the two groups. However, a significantly wider histological margin width was obtained in the AL group (10.7 vs. 6.9 mm, P<0.01). Major liver resection was performed more frequently in the PS group than in the AL group (P<0.001). Tables 1 and 2 summarise the perioperative results and the different types of liver resection performed, respectively.
Full table
Learning curve
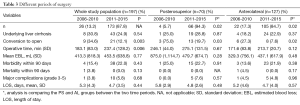
We performed significantly more PS LLR in the latter half of our experience when compared to the earlier period (2006-2010 vs. 2011-2015); a similar trend is also seen in the AL LLR (Figure 3). There is a significant improvement in our conversion rate in the whole cohort (P=0.003) as well as in each group (PS; AL: P=0.03; 0.02). Compared to our earlier experience [2006-2010], operative time for the whole cohort was significantly longer in the later experience [2011-2015] (237 vs. 183 min, P=0.006), although this difference was not significant when we analysed it in each group (PS/AL). There were no significant differences for the whole cohort and each group, in terms of blood loss, hospital stay, morbidity and mortality when comparing the earlier and later experience (Table 3).
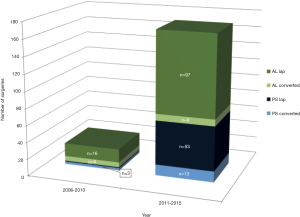
Full table
Although most of our resections were performed fully laparoscopically (n=175, 88.8%), some were assisted by various modalities e.g., hand-assist (n=6; 3.0%), laparoscopic-assisted (n=12; 6.1%), laparoscopic converted to hand-assist (n=1; 0.5%), robotic (n=3; 1.5%).
We also analysed cirrhosis as a factor in the PS group as these two factors (cirrhosis and difficult location) individually contribute to the difficulty of the resection as suggested by the difficulty score (15). There were no significant differences in perioperative outcomes such as operative time, blood loss, blood transfusion, conversion rate; there were no significant differences in morbidity and mortality either (Table 4).

Full table
Discussion
Advances in laparoscopic instruments, energy devices, stapler technology and the increasing experience have accelerated the utilization of LLR. Recently, it is estimated that from 1996 to 2014, a total of 5,388 LLR were reported to be performed including 1,184 major LLRs (16). Although minor LLR and laparoscopic left lateral sectionectomy are standard procedures in many institutions, major and difficult LLR are still limited to a few expert centres (11,17,18).
Benefits of LLR are well documented, in terms of reduced morbidity, less pain, shorter hospital stay, better cosmetic results and possibly improved immunologic and physiological outcomes (19-22). The disadvantages of LLR compared to open liver resection are less commonly discussed, these include poor visualization and manipulation of posterior and superior lesions and difficulty in bleeding control, especially for deep tumors in these less accessible portions of the liver (23). In fact, PS segment resections such as posterior sectionectomy are now more commonly considered and accepted as major or complex hepatectomies due to the complexity of the procedure, despite not satisfying the IHPBA Brisbane definition of major hepatectomy of three or more segments (10,18,24,25). Even non-anatomic LLR of small tumors in segments 7 and 8, where a limited volume of normal liver parenchyma is removed, can be technically challenging because the transection planes can be multiplanar, curved or angled especially if the lesions are deep or not easily accessible. A new scoring system to assess the difficulty of various laparoscopic hepatectomy procedures has been proposed based on 90 cases involving correlation of operators and external experts’ assessment combined with operative surrogates of difficulty such as blood loss and operative time. The difficulty score ranges from 1-10 based on five components: tumor location, extent of resection, tumor size, proximity to major vessels, and liver function (Child-Pugh) (Figure 4) (15). For example, procedures such as posterior sectionectomy and anatomical segment 7 resection will have at least a score of 9 or 8, respectively, depending on the other factors such as tumor size, proximity to major vessels and liver function (Figure 4).

Several studies have reported their outcomes of LLR for PS vs. AL lesions. Cho et al. reported in a retrospective review of their LLR experience with 36 patients with PS lesions and 92 with AL lesions. The mean operative time and the rate of intraoperative transfusion were significantly greater in the PS group than in the AL group but the mean hospital stay and the complication rates were similar in the two groups. Notably major liver resection was performed more frequently for the PS group than for the AL group (10). Similarly, the Oslo group compared LLR in same two groups (PS vs. AR, n=28 vs. 47) and reported equivalence in operative time, blood loss, tumor free margin, complications and hospital stay (26). Our results were similar and consistent with these studies, signifying PS LLR are technically more demanding but feasible and safe in well-selected patients (10,25,26). Since the initiation of the LLR program in our unit, we adopted a slow and cautious escalation in our selection of more difficult cases based on our learning curve and experience, this is demonstrated with significantly more PS LLR in the latter half of our experience when compared to the earlier period; a similar trend is also seen in the AL LLR (Figure 3). There is a significant improvement in our conversion rate in the whole cohort (P=0.003) as well as in each group (PS; AL: P=0.03; 0.02) without a significant difference in the hospital stay, morbidity and mortality rates (Table 3). Operative time was longer in the later experience for the whole cohort (P=0.006) and in each group (AL/PS), this is likely attributed to more complex resections being attempted and may be in part contributed by younger surgeons who returned to the department after their fellowships and started embarking on LLR in the later period as well. Of note, for the PS resections, blood loss also halved when compared to the earlier PS cases though this did not achieve statistical significance (likely due to small numbers in the earlier period). This represents an appropriate case selection and progression of our learning curve (Table 3). We previously analysed the factors for conversion in our center’s experience in minor LLR and demonstrated that individual surgeon and institution volume were the dominant risk factors for conversion during the learning curve. Based on conversion rates, the learning curve for an individual surgeon is about 15 to 20 cases and institution experience of at least 25 cases (11).
There are limited studies reporting laparoscopic LLR for PS tumors in the cirrhotic population. Xiang et al. reported their results in LLR for HCC in a cirrhotic population comparing HCC in the PS and AL locations (n=56 vs. 70). They noted although operative time, conversion rate, blood loss, transfusion rate and inflow occlusion time were significantly higher for the PS group, there were no differences in resection margins, complications, time to flatus and hospital stay in the two groups, leading to the conclusion that LLR for PS HCC in cirrhotic patients was safe and feasible (25). These were similar to our findings, albeit the cirrhotic patients in our study are limited to Child-Pugh A. PS lesions in difficult locations and moderate/severe cirrhosis were both considered relative contraindications for LLR, however, minimally invasive approaches provides a number of unique benefits in cirrhotic patients especially for limited resections (27). The smaller incisions cause less disruption of the abdominal wall collateral circulation. As complete evacuation of ascites is not necessary for a laparoscopic procedure, massive intraoperative fluid shifts can be minimised, contributing to the reduction in postoperative ascites seen with laparoscopy compared to open hepatectomy (28,29). Another advantage is the reduced amount of adhesions following laparoscopic surgery especially pertinent for patients undergoing resection of HCC, as salvage transplantation remains an important potential treatment for recurrences that are within the Milan criteria (30). This advantage is also appreciated in repeated resections not uncommon for patients with colorectal liver metastases. Belli et al. reported that in repeat liver resection after previous laparoscopic resection to be faster and safer, with less blood loss and risk of visceral injury when compared to previous open resection. Similarly, Laurent et al. reported that liver transplants following laparoscopic resection were performed in less time, with less blood loss and transfusion requirements when compared to prior open resections (19,31). In this study, it being our early experience, we selectively limited our LLR to non-cirrhotic and Child-Pugh A patients only. As suggested by the difficult scoring system, cirrhotic patients with PS segment may no doubt be technically more challenging than non-cirrhotic patients with AL lesions, but it is not associated with more morbidity or mortality in appropriately experienced hands (25) (Table 4).
The improved ability to tackle difficult segments in LLR safely can be attributed to many factors. Quality of preoperative imaging has improved and introduction of 3-dimensional (3D) reconstruction has enhanced pre- as well as intra-operative surgical planning (32). High resolution camera optics and 3D systems provide excellent definition and improve depth perception—one of the Achilles heel of laparoscopic surgery. New flexible laparoscopes further enable visualisation—previously difficult in the PS segments of the liver as well as over and around structures e.g., root of the right hepatic vein, dome of the right liver. Besides the advances in laparoscopic instrumentation and advent of advanced energy devices, experience has also augmented the technical ability of difficult LLR. Manoeuvres such as Belghiti’s hanging manoeuvre has been modified laparoscopically to aid difficult resections such as PS resections (33). In our experience, we found that thoracoscopic access via intercostal ports to approach segments 7 and 8 to be useful in certain situations, although the thoracoscopic approach can be associated with a longer operative time and increased risk of thoracic-related complications such as pneumothorax. If high thoracoscopic access is anticipated, two-lung intubation may be useful to minimise thoracic-related complications and a chest tube may be necessary prior to reversal of anaesthesia (26,34-36). Similarly, the hand-assisted approached has been advocated to facilitate LLR in PS segments by providing better retraction and a tactile component especially in the early phase of the learning curve. However, some do not find it particularly useful due to issues such as space constraints, interference with laparoscopic instruments, hand fatigue and troublesome air leakage to name a few (26,37,38). Positioning of the patient has also evolved from the traditional open surgery position such as supine or modified Lloyd-Davis to the established positions such as the “French position” for general LLR, or semi-/partial/full left lateral or even semi-prone position for laparoscopic resection of PS lesions to utilise gravity and to optimise surgical ergonomics (39).
Bleeding is a major concern and one of the main reasons for conversion in difficult resections such as PS lesions and in cirrhotic livers. Several factors can help mitigate these risks. Proper selection and familiarity with parenchymal transection devices is crucial. We found a combination of CUSA, advanced energy/bipolar devices, clips and staples to be useful in most situations. Proficient laparoscopic suturing skills are essential in timely haemostasis if initial attempts at hemostasis fail. To facilitate visualisation, temporarily increasing the pneumoperitioneum pressure to 15-20 mmHg can slow down the venous bleed enough to see the cause and site of the bleeding for precise hemostasis. In addition to the Pringle’s manoeuvre for inflow occlusion, especially in PS lesions, some surgeons recommend a tape around the root of the right hepatic vein ready for outflow occlusion if necessary (25).
Last but not least, we found maintaining the same dedicated team of circulating nurses, scrub nurses, camera operators, anaesthesia and a 2-surgeon team facilitated our learning experience. For example, the same group can learn faster from troubleshooting procedures, to faster set-up, optimal patient positioning and share useful tips and operative manoeuvres.
The retrospective, non-randomized nature of this study represents its biggest limitation. Patients with smaller, superficial tumors and in accessible parts of liver were more likely to be selected for LLR as compared to open surgery. We did not attempt LLR in Child-Pugh B and C patients partly because we are still on the learning curve and some of these patients may be better served by other treatment options such as liver transplantation or ablation if assessed to be suitable and appropriate. A variety of confounding factors also exist including surgeon factors such as individual surgeons’ comfort level for different levels of difficulty with regards to LLR and patient factors such as BMI, previous surgeries, severity of cirrhosis and tumor size/site/depth. In addition, evolution and changes in surgical techniques and equipment may have also confounded observations in this study. Nonetheless, prospective randomized trials are unlikely to be conducted to address the different approaches and lesions in different aspects of the liver. Future and larger studies can help to establish and guide the limits of LLR for difficult PS lesions in cirrhotic patients.
Conclusions
LLR appears to be safe and feasible in well-selected patients even in cirrhotic patients with difficult PS lesions, as long as a cautious learning curve is adopted. PS lesions may be technically more challenging than AL lesions and should be considered major hepatectomies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board approval was obtained.
References
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 1992;6:99.
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [PubMed]
- Vigano L, Laurent A, Tayar C, et al. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 2009;250:772-82. [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [PubMed]
- Dokmak S, Ftériche FS, Borscheid R, et al. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013;15:908-15. [PubMed]
- Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [PubMed]
- Cescon M, Vetrone G, Grazi GL, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg 2009;249:995-1002. [PubMed]
- Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-206; discussion 1206. [PubMed]
- Bryant R, Laurent A, Tayar C, et al. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg 2009;250:103-11. [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc 2008;22:2344-9. [PubMed]
- Goh BK, Chan CY, Wong JS, et al. Factors associated with and outcomes of open conversion after laparoscopic minor hepatectomy: initial experience at a single institution. Surg Endosc 2015;29:2636-42. [PubMed]
- Cho JY, Han HS, Yoon YS, et al. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 2008;144:32-8. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg 2013;257:377-82. [PubMed]
- Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci 2014;21:745-53. [PubMed]
- Dagher I, Gayet B, Tzanis D, et al. International experience for laparoscopic major liver resection. J Hepatobiliary Pancreat Sci 2014;21:732-6. [PubMed]
- Chang S, Laurent A, Tayar C, et al. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 2007;94:58-63. [PubMed]
- Dagher I, O'Rourke N, Geller DA, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg 2009;250:856-60. [PubMed]
- Belli G, Limongelli P, Fantini C, et al. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 2009;96:1041-8. [PubMed]
- Belli G, Fantini C, D'Agostino A, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc 2007;21:2004-11. [PubMed]
- Morino M, Morra I, Rosso E, et al. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc 2003;17:1914-8. [PubMed]
- Simillis C, Constantinides VA, Tekkis PP, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms--a meta-analysis. Surgery 2007;141:203-11. [PubMed]
- Kaneko H, Takagi S, Otsuka Y, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 2005;189:190-4. [PubMed]
- Di Fabio F, Samim M, Di Gioia P, et al. Laparoscopic major hepatectomies: clinical outcomes and classification. World J Surg 2014;38:3169-74. [PubMed]
- Xiang L, Xiao L, Li J, et al. Safety and Feasibility of Laparoscopic Hepatectomy for Hepatocellular Carcinoma in the Posterosuperior Liver Segments. World J Surg 2015;39:1202-9. [PubMed]
- Kazaryan AM, Rosok BI, Marangos IP, et al. Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc 2011;25:3881-9. [PubMed]
- Cannon RM, Buell JF. Laparoscopic Liver Resection. In: Elgeidie AA, editor. Updated Topics in Minimally Invasive Abdominal Surgery. Rijeka: InTech, 2011:15.
- Dagher I, Di Giuro G, Dubrez J, et al. Laparoscopic versus open right hepatectomy: a comparative study. Am J Surg 2009;198:173-7. [PubMed]
- Gigot JF, Glineur D, Santiago Azagra J, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg 2002;236:90-7. [PubMed]
- Lee SY, Konstantinidis IT, Eaton AA, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford) 2014;16:943-53. [PubMed]
- Laurent A, Tayar C, Andréoletti M, et al. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2009;16:310-4. [PubMed]
- Lamata P, Lamata F, Sojar V, et al. Use of the Resection Map system as guidance during hepatectomy. Surg Endosc 2010;24:2327-37. [PubMed]
- Casaccia M, Andorno E, Di Domenico S, et al. Laparoscopic right posterior sectionectomy for hepatocellular carcinoma using a modified liver-hanging maneuver. J Laparoendosc Adv Surg Tech A 2012;22:488-91. [PubMed]
- Lee W, Han HS, Yoon YS, et al. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci 2014;21:E65-8. [PubMed]
- Teramoto K, Kawamura T, Takamatsu S, et al. Laparoscopic and thoracoscopic approaches for the treatment of hepatocellular carcinoma. Am J Surg 2005;189:474-8. [PubMed]
- Chiow AK, Lewin J, Manoharan B, et al. Intercostal and transthoracic trocars enable easier laparoscopic resection of dome liver lesions. HPB (Oxford) 2015;17:299-303. [PubMed]
- Herman P, Kruger JA, Perini MV, et al. Laparoscopic hepatic posterior sectionectomy: a hand-assisted approach. Ann Surg Oncol 2013;20:1266. [PubMed]
- Dulucq JL, Wintringer P, Stabilini C, et al. Laparoscopic liver resections: a single center experience. Surg Endosc 2005;19:886-91. [PubMed]
- Ikeda T, Toshima T, Harimoto N, et al. Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc 2014;28:2484-92. [PubMed]


